Xenografts is the fastest growing segment, North America is the largest market globally
Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key Market Drivers
Technological advancements in biomaterials and manufacturing fundamentally reshape the capabilities of biological implants, leading to products that offer superior integration, longevity, and patient-specific solutions. Ongoing research in advanced biomaterials, including novel polymers and surface-modified metals, enhances biocompatibility and functionality, thereby reducing adverse reactions and improving implant performance. Advanced manufacturing techniques, particularly 3D printing, enable the creation of highly customized implants with intricate designs that closely mimic natural biological structures, fostering better osseointegration and tissue regeneration.Key Market Challenges
The complex and often prolonged regulatory approval processes represent a significant impediment to the expansion of the global biological implants market. These stringent requirements impose substantial financial burdens and extend development timelines for manufacturers. The necessity to adhere to evolving regulations, particularly evident in regions with advanced regulatory frameworks, escalates costs associated with clinical evaluations, post-market surveillance, and certification. This increased regulatory load directly curtails manufacturers' ability to allocate resources to research and development of novel biological implants.Key Market Trends
A key trend is the increased focus on regenerative bio-implants, which aim to restore, replace, or regenerate damaged tissues and organs through biological mechanisms, moving beyond mere mechanical replacement. This involves developing implants that integrate advanced biomaterials with cellular components or growth factors to actively stimulate the body's natural healing processes. For instance, REGENXBIO Inc. and Nippon Shinyaku Co., Ltd. announced a strategic partnership in January 2025 for the development and commercialization of gene therapies, with REGENXBIO receiving $110 million upfront and up to an additional $700 million in potential milestones. This significant investment, as reported by Contract Pharma, January 15, 2025, in "Regenxbio, Nippon Shinyaku Partner for MPS II, MPS I Gene Therapies," highlights the market's trajectory towards biological solutions that foster genuine tissue regeneration.Key Market Players Profiled:
- Integra LifeSciences Corporation
- NuVasive, Inc.
- Edwards Lifesciences Corporation
- Stryker Corp.
- LifeCell International Pvt. Ltd.
- Medtronic Plc
- Osteotec Limited
- BioPolymer GmbH & Co KG
- Johnson & Johnson Services, Inc
- Baxter International Inc.
Report Scope:
In this report, the Global Biological Implants Market has been segmented into the following categories:By Product:
- Autografts
- Allografts
- Xenografts
By Application:
- Cardiovascular Implants
- Orthopedic Implants
- Others Soft Tissue Implants
By Region:
- North America
- Asia Pacific
- Europe
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Biological Implants Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Integra LifeSciences Corporation
- NuVasive, Inc.
- Edwards Lifesciences Corporation
- Stryker Corp.
- LifeCell International Pvt. Ltd.
- Medtronic Plc
- Osteotec Limited
- BioPolymer GmbH & Co KG
- Johnson & Johnson Services, Inc
- Baxter International Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | November 2025 |
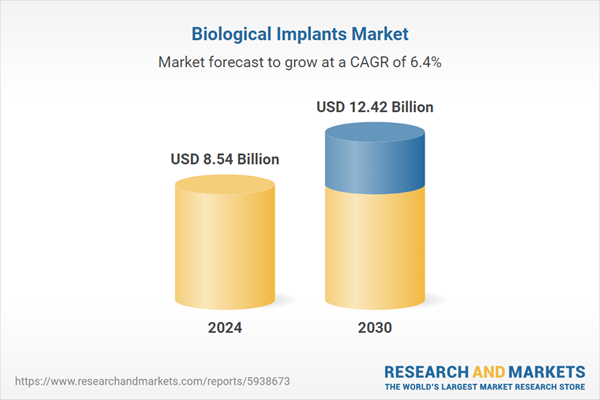
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 8.54 Billion |
| Forecasted Market Value ( USD | $ 12.42 Billion |
| Compound Annual Growth Rate | 6.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |