Increased Awareness Regarding Early Diagnosis of Breast Cancer
This report comes with 10% free customization, enabling you to add data that meets your specific business needs.
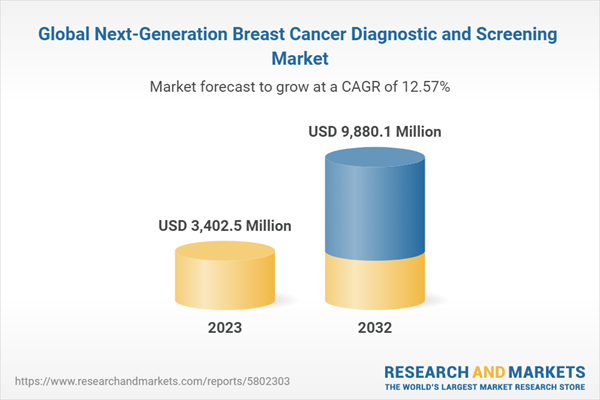
The global next-generation breast cancer diagnostic and screening market was valued at $3,137.4 million in 2022 and is anticipated to reach $9,880.1 million by 2032, witnessing a CAGR of 12.57% during the forecast period 2023-2032. The growth in the global next-generation breast cancer diagnostic and screening market is expected to be driven by the increase in the prevalence of breast cancer cases globally, rising awareness regarding early diagnosis of breast cancer, growing focus on breast cancer biomarkers for effective screening, prognosis, and personalized treatment, and increasing partnerships and collaborations amongst market players.
Market Lifecycle Stage
The next-generation breast cancer diagnostic and screening market is in the developing phase. Technological advancements in the development of next-generation breast cancer diagnostics and the growing scope for molecular breast cancer diagnosis techniques in emerging countries can be some of the major opportunities in the global next-generation breast cancer diagnostic and screening market. Furthermore, some of the key trends going on in the market are the implementation of liquid biopsy for breast cancer diagnosis and the complete automation of next-generation techniques.
Impact of COVID-19
COVID-19 had a significant impact on the global next-generation breast cancer diagnostic and screening market. There was a significant shift observed toward research activities for treating and diagnosing COVID-19, which resulted in a halt in research activities for next-generation breast cancer diagnostic and screening. Moreover, the market players witnessed a loss in 2020 due to COVID-19 restrictions and prevention actions such as a reduction in supply chain and sales.
However, since the situations are now almost back to normal, the next-generation breast cancer diagnostic and screening market is expected to overcome all the impact of COVID-19 during the forecast period 2023-2032.
Market Segmentation
Segmentation 1: by Technology
- Real-Time PCR
- Immunohistochemistry (IHC)
- Next-Generation Sequencing (NGS)
- Fluorescence In-Situ Hybridization (FISH)
- Others
Based on technology, the global next-generation breast cancer diagnostic and screening market is expected to be dominated by the immunohistochemistry (IHC) segment.
Segmentation 2: by Biomarker
- BRCA1/2
- ER/PR Receptors
- HER-2
- Others
Based on biomarker, the global next-generation breast cancer diagnostic and screening market is dominated by the BRCA1/2 segment.
Segmentation 3: by Cancer Sub-Type
- Luminal A
- Luminal B
- Triple Negative/Basal Like
- Human Epidermal Growth Factor Receptor 2 (HER-2) Enriched
Based on cancer sub-type, the global next-generation breast cancer diagnostic and screening market is dominated by the luminal A segment.
Segmentation 4: by Offering
- Products
- Services
Based on offering, the global next-generation breast cancer diagnostic and screening market is dominated by the services segment.
Segmentation 5: by End User
- Hospitals and Clinics
- Diagnostic Centers and Reference Labs
- Academic and Research Institutes
Based on end user, the global next-generation breast cancer diagnostic and screening market is dominated by the hospitals and clinics segment.
Segmentation 6: by Region
- North America - U.S., Canada
- Europe - Germany, France, U.K., Italy, Spain, and Rest-of-Europe
- Asia-Pacific - Japan, China, India, Australia, South Korea, Singapore, and Rest-of-Asia-Pacific
- Latin America - Brazil, Mexico, and Rest-of-Latin America
- Rest-of-the-World
Based on region, the global next-generation breast cancer diagnostic and screening market is dominated by North America.
Recent Developments in the Global Next-Generation Breast Cancer Diagnostic and Screening Market
- In April 2023, Syantra Inc., a Canada-based company, received CE mark approval for its Syantra DX breast cancer test.
- In March 2023, NeoGenomics, Inc. launched its RaDaR assay, which is a liquid biopsy test for minimal residual disease (MRD).
- In February 2023, HALO Diagnostics launched its new HALO pathway for early breast cancer detection.
- In January 2023, Agilent Technologies, Inc. acquired Avida Biomed, a company that develops high-performance NGS target enrichment workflows for cancer research.
- In October 2022, Lucence Diagnostics Pte Ltd entered into a distribution partnership with Omnigen, under which Omnigen would distribute Lucence Diagnostics Pte Ltd’s LiquidHALLMARK assay in Turkey.
- In March 2022, Myriad Genetics, Inc. received the U.S. FDA approval for its BRACanalysis CDx for being used as a companion diagnostic test and for the identification of breast cancer patients who may benefit from Lynparza in early breast cancer stages.
- In February 2022, Laboratory Corporation of America Holdings acquired Personal Genome Diagnostics Inc., a company that deals in cancer genomics and provides an assortment of comprehensive liquid biopsy and tissue-based products.
- In January 2022, Agendia Inc. entered into a partnership with Illumina Inc. to expand genomic testing for breast cancer care.
- In December 2021, Exai Bio, Inc. raised funding of $67.5 million as a part of its Series A funding round. The funding was in order to bring advancement in the next-generation RNA-based liquid biopsy platform of the company, which is indicated for early detection of cancer.
- In June 2021, Veracyte, Inc. acquired HalioDx for an amount of $318 million to expand its portfolio in cancer in-vitro diagnostics.
- In May 2021, Hologic, Inc. acquired Biotheranostics, Inc., a company that provides molecular diagnostic tests for breast and metastatic cancers.
- In November 2020, Agilent Technologies, Inc. received U.S. FDA approval for its PD-L1 IHC 22C3 pharmDx for triple-negative breast cancer.
Demand - Drivers and Limitations
The following are the drivers for the global next-generation breast cancer diagnostic and screening market:
- Increasing Prevalence of Breast Cancer Globally
- Increased Awareness Regarding Early Diagnosis of Breast Cancer
- Growing Focus on Breast Cancer Biomarkers for Effective Screening, Prognosis, and Personalized Treatment
- Increasing Partnerships and Collaborations amongst Market Players
The market is expected to face some limitations as well due to the following restraints:
- High Cost of Tests and Requirement of High Capital Investment for Testing Set-Up
- Occurrence of False Positive and False Negative Results
How can this report add value to an organization?
Product/Innovation Strategy: The technology segment helps the reader understand the different types of technologies being used in next-generation breast cancer diagnosis and screening. Moreover, the study provides the reader with a detailed understanding of the next-generation breast cancer diagnostic and screening market based on biomarkers (BRCA1/2, ER/PR receptors, HER-2, and others), cancer sub-type (luminal A, luminal B, triple negative/basal-like, and HER-2 enriched), and offerings (products and services).
Growth/Marketing Strategy: The global next-generation breast cancer diagnostic and screening market has seen major development by key players operating in the market, such as business expansions, partnerships, collaborations, mergers and acquisitions, product launches, and funding activities. Partnerships, alliances, and business expansions accounted for the maximum number of key developments in the global next-generation breast cancer diagnostic and screening market, followed by regulatory and legal activities and mergers and acquisitions.
Competitive Strategy: Key players in the global next-generation breast cancer diagnostic and screening market analyzed and profiled in the study involve players that offer next-generation breast cancer diagnostic and screening products and services. Moreover, comprehensive competitive strategies such as partnerships, agreements, collaborations, product launches and approvals, and funding scenarios will aid the reader in understanding the untapped revenue pockets in the market.
Key Market Players and Competition Synopsis
The companies that are profiled have been selected based on inputs gathered from primary experts and analyzing the company’s coverage, product portfolio, and market penetration.
Key Companies Profiled
- Abbott Laboratories
- Agendia Inc.
- Agilent Technologies, Inc.
- Bio-Rad Laboratories, Inc.
- BGI Genomics Co., Ltd.
- CENTOGENE N.V.
- Danaher Corporation
- Exact Sciences Corporation
- F. Hoffmann-La Roche Ltd.
- Illumina, Inc.
- Laboratory Corporation of America Holdings
- Thermo Fisher Scientific Inc.
- Lucence Diagnostics Pte Ltd
- Myriad Genetics, Inc.
- Natera, Inc.
Table of Contents
Companies Mentioned
- Abbott Laboratories
- Agendia Inc.
- Agilent Technologies, Inc.
- Bio-Rad Laboratories, Inc.
- BGI Genomics Co., Ltd.
- CENTOGENE N.V.
- Danaher Corporation
- Exact Sciences Corporation
- F. Hoffmann-La Roche Ltd.
- Illumina, Inc.
- Laboratory Corporation of America Holdings
- Thermo Fisher Scientific Inc.
- Lucence Diagnostics Pte Ltd
- Myriad Genetics, Inc.
- Natera, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 244 |
| Published | May 2023 |
| Forecast Period | 2023 - 2032 |
| Estimated Market Value ( USD | $ 3402.5 Million |
| Forecasted Market Value ( USD | $ 9880.1 Million |
| Compound Annual Growth Rate | 12.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |