The China market dominated the Asia Pacific Competent Cells Market by Country in 2023, and is forecast to continue being a dominant market till 2031; thereby, achieving a market value of $304.9 million by 2031. The Japan market is registering a CAGR of 7.9% during (2024 - 2031). Additionally, The India market would showcase a CAGR of 9.3% during (2024 - 2031).
Academic and research laboratories represent significant users of these cells. These institutions rely on these cells for fundamental molecular biology, genetics, and biotechnology research. Adoption in academia is driven by the need for tools that facilitate gene cloning, gene editing, and the construction of genetic libraries for studying gene function and disease mechanisms. Continuous advancements in genetic engineering, synthetic biology, and high-throughput screening technologies drive adoption.
Additionally, competent cell suppliers offer products tailored to specific research and industrial applications. Customization options include specialized strains optimized for high transformation efficiency, protein expression, or specific genetic modifications. This diversity allows researchers to choose products that best meet their experimental requirements.
India’s biotech sector is expanding rapidly, focusing on developing and producing biopharmaceuticals, including vaccines, therapeutic proteins, and biosimilars. As per the National Investment Promotion & Facilitation Agency, India's bioeconomy sector, valued at $137 billion in 2023, is set for significant expansion, with estimates indicating it will reach $150 billion by 2025 and $300 billion by 2030. This growth reflects a strong annual growth rate of 17%. The country boasts formidable capabilities in contract manufacturing, research, and clinical trials, bolstered by the presence of the highest number of US FDA-approved plants globally outside the US.
Additionally, The Chinese government fosters biopharmaceutical innovation through initiatives like the Made in China 2025 strategy and regulatory reforms to streamline drug approval processes. According to the International Trade Administration (ITA), China’s pharmaceutical sector has constantly grown and reached $161.8 billion in 2023, taking about 30% of the global industry share. Suppliers offering compliant and validated competent cell products gain a competitive advantage, supporting local and multinational pharmaceutical firms in achieving regulatory approvals and market entry in China. Thus, the growing biotech and pharmaceutical industries in the region are driving the market's growth.
List of Key Companies Profiled
- Thermo Fisher Scientific, Inc.
- Merck KGaA
- Agilent Technologies, Inc.
- Bio-Rad Laboratories, Inc.
- Promega Corporation
- New England Biolabs, Inc.
- Qiagen N.V
- Hoffmann-La Roche Ltd.
- Takara Bio Inc. (Takara Holdings Inc.)
- Lonza Group Ltd. (Capsugel)
Market Report Segmentation
By Type- Chemically Competent Cells
- Electrocompetent Cells
- Ultracompetent Cells
- Cloning
- Protein Expression
- Mutagenesis
- Others
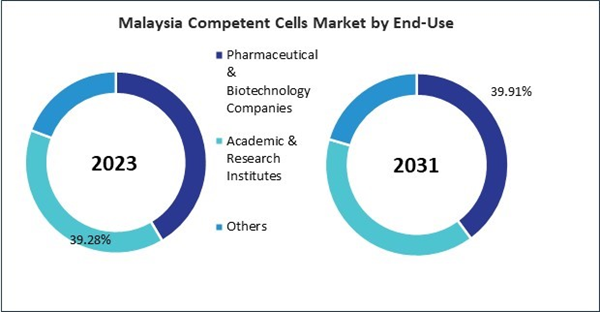
- Pharmaceutical & Biotechnology Companies
- Academic & Research Institutes
- Others
- China
- Japan
- India
- South Korea
- Australia
- Malaysia
- Rest of Asia Pacific
Table of Contents
Companies Mentioned
- Thermo Fisher Scientific, Inc.
- Merck KGaA
- Agilent Technologies, Inc.
- Bio-Rad Laboratories, Inc.
- Promega Corporation
- New England Biolabs, Inc.
- Qiagen N.V
- F. Hoffmann-La Roche Ltd.
- Takara Bio Inc. (Takara Holdings Inc.)
- Lonza Group Ltd. (Capsugel)