The China market dominated the Asia Pacific Rapid Microbiology Testing Market by Country in 2023, and would continue to be a dominant market till 2031; thereby, achieving a market value of $792.4 million by 2031. The Japan market is registering a CAGR of 10.6% during (2024 - 2031). Additionally, The India market would showcase a CAGR of 12% during (2024 - 2031).
Unnecessary antibiotic use is a significant driver of antimicrobial resistance, contributing to the proliferation of drug-resistant pathogens and the erosion of antibiotic effectiveness. This testing aids in the timely identification of infectious agents and their susceptibility to antibiotics, allowing healthcare providers to differentiate between bacterial and viral infections and avoid inappropriate antibiotic prescribing. By providing actionable diagnostic information within hours rather than days, rapid testing technologies empower healthcare providers to make evidence-based decisions regarding antibiotic therapy, reducing the overuse and misuse of antibiotics and preserving their effectiveness for future generations.
Furthermore, continuous innovation and technology developments to improve the speed, accuracy, and accessibility of infectious disease diagnoses characterize the market. Molecular diagnostics technologies like nucleic acid amplification assays are at the forefront of this testing innovation. Polymerase chain reaction, loop-mediated isothermal amplification, and nucleic acid sequencing platforms enable the rapid and sensitive detection of microbial nucleic acids, offering high specificity and multiplexing capabilities for identifying multiple pathogens in a single test.
Pharmaceutical manufacturers in India rely on these testing solutions for in-process monitoring, environmental monitoring, and finished product testing throughout the manufacturing process. Rapid testing technologies facilitate the timely detection of microbial contaminants, including bacteria, fungi, and endotoxins, in raw materials, intermediate products, and finished pharmaceutical formulations. As per the data released in 2023 by the India Brand Equity Foundation, it is expected that the Indian pharmaceuticals sector will attain a value of US$65 billion by 2024 and US$130 billion by 2030. The Indian pharmaceutical industry is estimated to be worth around $50 billion, of which more than $25 billion is derived from exports, according to government data. Therefore, with the growing pharmaceutical sector in Asia Pacific, there will be increased demand in the region.
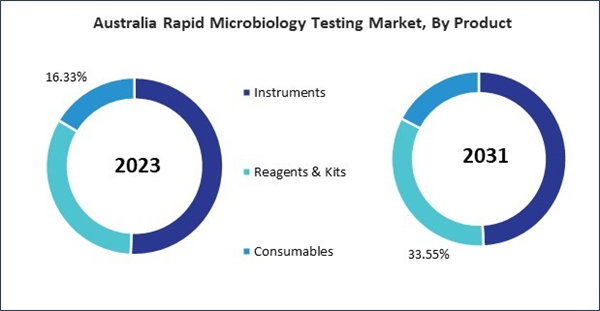
Based on Method, the market is segmented into Growth-based Rapid Microbiology Testing, Cellular Component-based Rapid Microbiology Testing, Nucleic Acid-based Rapid Microbiology Testing, Viability-based Rapid Microbiology Testing and Others. Based on Application, the market is segmented into Industrial Testing (Food & Beverage Testing, Pharmaceutical & Biological Drug Testing, Environmental Testing, Cosmetics & Personal Care Products Testing and Others), Clinical Disease Diagnosis and Research Applications. Based on Product, the market is segmented into Instruments (Automated Microbial Identification & Antimicrobial Susceptibility Testing Systems, Mass Spectrometers, PCR Systems, Bioluminescence & Fluorescence-based Detection Systems, Cytometers, Active Air Samplers and Others), Reagents & Kits, and Consumables. Based on countries, the market is segmented into China, Japan, India, South Korea, Australia, Malaysia, and Rest of Asia Pacific.
List of Key Companies Profiled
- BioMerieux S.A.
- Becton, Dickinson and Company
- Danaher Corporation
- Abbott Laboratories
- Thermo Fisher Scientific, Inc.
- Merck KGaA
- Bruker Corporation
- QuidelOrtho Corporation
- Charles River Laboratories International, Inc.
- Neogen Corporation
Market Report Segmentation
By Method- Growth-based Rapid Microbiology Testing
- Cellular Component-based Rapid Microbiology Testing
- Nucleic Acid-based Rapid Microbiology Testing
- Viability-based Rapid Microbiology Testing
- Others
- Industrial Testing
- Food & Beverage Testing
- Pharmaceutical & Biological Drug Testing
- Environmental Testing
- Cosmetics & Personal Care Products Testing
- Others
- Clinical Disease Diagnosis
- Research Applications
- Instruments
- Automated Microbial Identification & Antimicrobial Susceptibility Testing Systems
- Mass Spectrometers
- PCR Systems
- Bioluminescence & Fluorescence-based Detection Systems
- Cytometers
- Active Air Samplers
- Others
- Reagents & Kits
- Consumables
- China
- Japan
- India
- South Korea
- Australia
- Malaysia
- Rest of Asia Pacific
Table of Contents
Companies Mentioned
- BioMerieux S.A.
- Becton, Dickinson and Company
- Danaher Corporation
- Abbott Laboratories
- Thermo Fisher Scientific, Inc.
- Merck KGaA
- Bruker Corporation
- QuidelOrtho Corporation
- Charles River Laboratories International, Inc.
- Neogen Corporation