The China market dominated the Asia Pacific Regulatory Information Management System Market by Country in 2023, and would continue to be a dominant market till 2031; thereby, achieving a market value of $347.8 Million by 2031. The Japan market is experiencing a CAGR of 10.7% during (2024 - 2031). Additionally, The India market would exhibit a CAGR of 12% during (2024 - 2031).
Regulatory information management system (RIMS) facilitates regulatory submissions and approvals, monitor regulatory requirements, and manage documentation to assist organizations in ensuring compliance with applicable standards and regulations. RIMS centralizes storing and managing regulatory documents, such as product dossiers, submissions, licenses, and certificates. They enable version control, document tracking, and access control to ensure data integrity and security.
RIMS streamlines the process of preparing and submitting regulatory documents to regulatory authorities. They facilitate the assembly, formatting, and submission of regulatory filings, track submission status, and manage communication with regulatory agencies. RIMS helps companies manage changes in regulatory requirements, such as updates to laws, standards, or guidelines. They enable tracking regulatory changes, impact assessment, and implementation of necessary updates to ensure ongoing compliance.
China's pharmaceutical industry has experienced rapid growth in recent years, fueled by increasing healthcare expenditures, an aging population, and government initiatives to promote domestic innovation and manufacturing. As the pharmaceutical sector expands, the complexity of regulatory compliance requirements also increases, driving the demand for robust regulatory information management systems to ensure compliance with regulatory standards, streamline regulatory processes, and accelerate product approvals in China. As per China.org, in 2021, the combined business revenue of China’s pharmaceutical companies increased by 18.7 percent annually, the greatest growth rate in five years. Therefore, due to the above-mentioned factors, the market will grow significantly in this region.
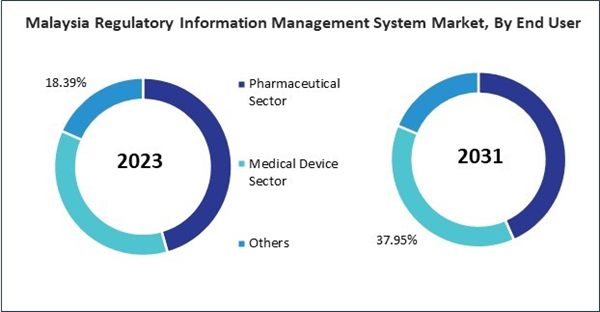
Based on End User, the market is segmented into Pharmaceutical Sector, Medical Device Sector, and Others. Based on countries, the market is segmented into China, Japan, India, South Korea, Australia, Malaysia, and Rest of Asia Pacific.
List of Key Companies Profiled
- Veeva Systems, Inc.
- Korber AG (Optel Group)
- ArisGlobal LLC (Nordic Capital Limited)
- Calyx
- Ennov SAS
- MasterControl, Inc.
- LORENZ Life Sciences Group
- AmpleLogic
- Cencora, Inc. (PharmaLex Holding GmbH)
- Ithos Global Inc. (Cordance Group)
Market Report Segmentation
By End User- Pharmaceutical Sector
- Medical Device Sector
- Others
- China
- Japan
- India
- South Korea
- Australia
- Malaysia
- Rest of Asia Pacific
Table of Contents
Companies Mentioned
- Veeva Systems, Inc.
- Korber AG (Optel Group)
- ArisGlobal LLC (Nordic Capital Limited)
- Calyx
- Ennov SAS
- MasterControl, Inc.
- LORENZ Life Sciences Group
- AmpleLogic
- Cencora, Inc. (PharmaLex Holding GmbH)
- Ithos Global Inc. (Cordance Group)