Low-Cost Surgery Under Medical Tourism Fuels the Asia Pacific Spinal Fusion Devices Market
Spinal fusion surgeries are much costlier; many patients slide back from the decision to undergo spinal surgeries. Also, in many cases, the cost of spinal implants is not covered under health insurance plans, limiting the number of surgeries. On the other hand, medical tourism has enabled to offer spinal fusion surgeries at cheaper costs. Countries in developing regions have significantly developed medical tourism and have advanced technologies that provide world-class medical services at cheaper rates. In India, it is nearly between US$ 8000 and US$ 12,000. Also, the cost of thoracoplasty in India is between US$ 2,500 and US$ 4,000. Offering critical surgeries at cheaper costs increases patient flow to these countries for their medical treatments. Also, the availability of advanced medical techniques and increasing government funding to increase medical tourism are leading to the increasing demand for advanced surgical implants. Further, the advancing healthcare infrastructure in developing countries will likely continue the demand for advanced medical devices, enhancing growth opportunities for the Asia Pacific spinal fusion devices market.Asia Pacific Spinal Fusion Devices Market Overview
The government of China has introduced various policies to support the development of the medical devices, including the spine surgery devices. These policies aim to improve product quality, promote domestic manufacturing, and ensure patient safety. Additionally, the government has been working on healthcare reforms to enhance access to healthcare services, which indirectly benefits the spine market by increasing the number of patients seeking treatment. The Chinese market has its own regulatory environment and unique considerations. Companies are focusing to enter the Chinese spinal surgery devices market. These companies need to comply with the country’s regulatory requirements, including registration and approval processes. For instance, in June 2023, the technical guidelines for clinical evaluation of spinal fusion devices were issued by National Medical Products Administration (NMPA) and according to these guidelines spinal fusion devices were categorized under Class III medical devices.Moreover, with the increasing technological advancements and product innovations, mid-size to smaller companies’ are increasing their market presence by introducing new technologies with better usability. For instance, in March 2021, Chinese authorities issued an approval for orthopedic surgical robot which carried out a spinal fusion surgery and a discectomy in a patient in Shenzhen.
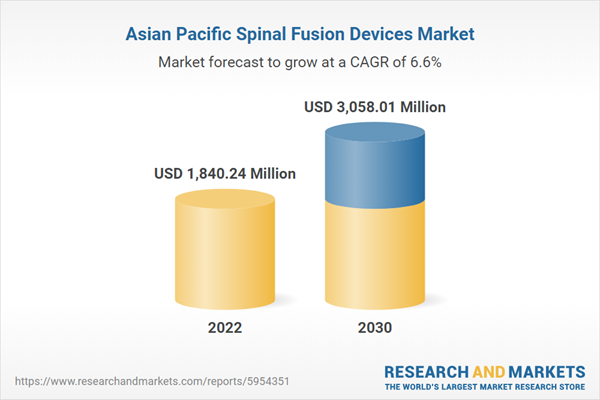
Asi
a Pacific Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Million)
Asia Pacific Spinal Fusion Devices Market Segmentation
The Asia Pacific spinal fusion devices market is segmented based on product type, surgery type, disease indications, end user, and country.Based on product type, the Asia Pacific spinal fusion devices market is segmented into thoracolumbar devices, cervical fixation devices, and interbody fusion devices. The thoracolumbar devices segment held the largest share in 2022.
By surgery type, the Asia Pacific spinal fusion devices market is bifurcated into open spine surgery and minimally invasive spine surgery. The open spine surgery segment held the largest share in 2022.
By disease indications, the Asia Pacific spinal fusion devices market is segmented into degenerative disc, trauma and fractures, complex deformity, and others. The degenerative disc segment held the largest share in 2022.
In terms of end users, the Asia Pacific spinal fusion devices market is categorized into hospitals, specialty clinics, and others. The hospitals segment held the largest share in 2022.
Based on country, the Asia Pacific spinal fusion devices market is segmented into China, Japan, India, Australia, South Korea, and the Rest of Asia Pacific. China dominated the Asia Pacific spinal fusion devices market in 2022.
ATEC Spine Inc, B. Braun SE, DePuy Synthes Inc, Globus Medical Inc, Medtronic Plc, NuVasive Inc, Stryker Corp, and ZimVie Inc are some of the leading companies operating in the Asia Pacific spinal fusion devices market.
Reasons to Buy
- Save and reduce time carrying out entry-level research by identifying the growth, size, leading players, and segments in the Asia Pacific spinal fusion devices market.
- Highlights key business priorities in order to assist companies to realign their business strategies
- The key findings and recommendations highlight crucial progressive industry trends in the Asia Pacific spinal fusion devices market, thereby allowing players across the value chain to develop effective long-term strategies
- Develop/modify business expansion plans by using substantial growth offering developed and emerging markets
- Scrutinize in-depth Asia Pacific market trends and outlook coupled with the factors driving the spinal fusion devices market, as well as those hindering it
- Enhance the decision-making process by understanding the strategies that underpin commercial interest with respect to client products, segmentation, pricing, and distribution
Table of Contents
Companies Mentioned
- ATEC Spine Inc
- B. Braun SE
- DePuy Synthes Inc
- Globus Medical Inc
- Medtronic Plc
- NuVasive Inc
- Stryker Corp
- ZimVie Inc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 113 |
| Published | January 2024 |
| Forecast Period | 2022 - 2030 |
| Estimated Market Value ( USD | $ 1840.24 Million |
| Forecasted Market Value ( USD | $ 3058.01 Million |
| Compound Annual Growth Rate | 6.6% |
| Regions Covered | Asia Pacific |
| No. of Companies Mentioned | 8 |