The China market dominated the Asia Pacific Tuberculosis Diagnostics Market by Country in 2023, and would continue to be a dominant market till 2031; thereby, achieving a market value of $312.6 million by 2031. The Japan market is registering a CAGR of 4.5% during (2024 - 2031). Additionally, The India market would showcase a CAGR of 5.9% during (2024 - 2031).
The market is driven by the need for timely and accurate disease detection, crucial for reducing transmission, initiating effective treatment, and improving patient outcomes. Early diagnosis of TB is essential because it allows for the prompt commencement of treatment, thereby reducing the likelihood of transmission to others and minimizing the risk of complications. This is particularly important in the case of drug-resistant TB, where delays in diagnosis can lead to the spread of more virulent strains that are harder to treat.
Moreover, the market is influenced by a variety of health initiatives and policies that are designed to alleviate the burden of TB, in addition to technological advancements. These initiatives have spurred investment in TB diagnostics from public health agencies and private sector companies, leading to the development of new tools and technologies designed to improve TB detection and management.
In China, the demand for this is rising due to the country’s ongoing public health efforts to control TB, particularly in regions with high incidence rates and among vulnerable populations. The Chinese government has prioritized TB detection and treatment, especially in western provinces with less developed healthcare infrastructure. Implementing the National TB Control Program has increased the use of advanced diagnostic methods, including nucleic acid amplification tests (NAATs) and digital radiography, to ensure early and accurate diagnosis. Across the region, there is a clear trend towards adopting innovative diagnostic tools, supported by government initiatives, international funding, and public health campaigns to reduce TB incidence and mortality. Hence, the Asia Pacific region’s diverse and dynamic market is poised for continued growth as these nations intensify their efforts to combat TB and expand access to effective diagnostics.
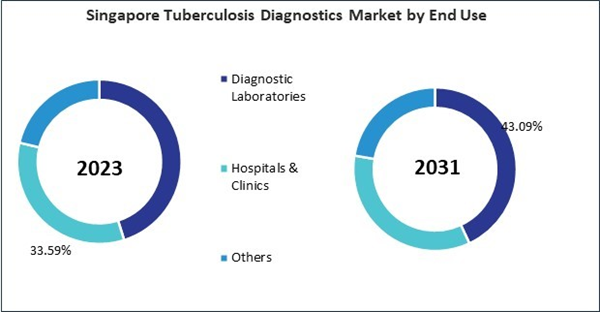
Based on End Use, the market is segmented into Diagnostic Laboratories, Hospitals & Clinics, and Others. Based on Type, the market is segmented into Detection of Latent Infection (Skin Test & IGRA), Phage Assay, Detection of Drug Resistance (DST), Nucleic Acid Testing, Radiographic Method, Cytokine Detection Assay, Diagnostic Laboratory Methods, and Other Methods. Based on countries, the market is segmented into China, Japan, India, South Korea, Singapore, Malaysia, and Rest of Asia Pacific.
List of Key Companies Profiled
- Abbott Laboratories
- Qiagen N.V
- Thermo Fisher Scientific Inc.
- Becton, Dickinson and Company
- F.Hoffmann-La Roche Ltd.
- Hologic, Inc.
- Danaher Corporation
- DiaSorin S.p.A.
- Bruker Corporation
Market Report Segmentation
By End Use- Diagnostic Laboratories
- Hospitals & Clinics
- Others
- Detection of Latent Infection (Skin Test & IGRA)
- Phage Assay
- Detection of Drug Resistance (DST)
- Nucleic Acid Testing
- Radiographic Method
- Cytokine Detection Assay
- Diagnostic Laboratory Methods
- Other Methods
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
Table of Contents
Companies Mentioned
- Abbott Laboratories
- Qiagen N.V
- Thermo Fisher Scientific Inc.
- Becton, Dickinson and Company
- F. Hoffmann-La Roche Ltd.
- Hologic, Inc.
- Danaher Corporation
- DiaSorin S.p.A.
- Bruker Corporation
- Revvity, Inc.