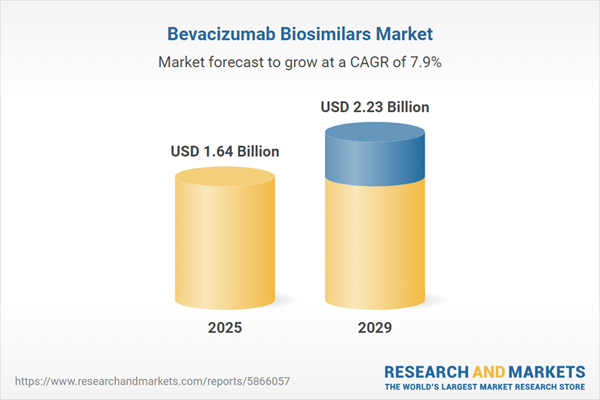
The bevacizumab biosimilars market size is expected to see strong growth in the next few years. It will grow to $2.23 billion in 2029 at a compound annual growth rate (CAGR) of 7.9%. The growth in the forecast period can be attributed to rising demand for cancer therapies, biosimilar development expertise, healthcare system pressures, biosimilar regulatory landscape, biosimilar interchangeability. Major trends in the forecast period include collaborations and partnerships, regulatory advancements and approvals, biosimilar development and innovation, market access strategies, biosimilar lifecycle management.
The forecast of 7.9% growth over the next five years reflects a modest reduction of 0.2% from the previous estimate for this market. This reduction is primarily due to the impact of tariffs between the US and other countries. Tariff escalations are likely to burden U.S. oncology clinics by driving up costs of bevacizumab biosimilars (e.g., Mvasi, Zirabev) sourced from India and South Korea, exacerbating metastatic cancer treatment expenses and increasing barriers to affordable anti-angiogenesis therapy for colorectal, lung, and ovarian cancer patients. The effect will also be felt more widely due to reciprocal tariffs and the negative effect on the global economy and trade due to increased trade tensions and restrictions.
The increasing incidence of cancer cases is anticipated to drive the growth of the bevacizumab biosimilars market in the future. Cancer incidence refers to the number of new cancer cases in a defined population over a specific period. Bevacizumab is a biologic that inhibits tumor angiogenesis, potentially leading to tumor shrinkage and growth inhibition. It is comparable to reference biologics in terms of efficacy and safety for treating patients with advanced non-small cell lung cancer or metastatic colorectal cancer. For example, in October 2024, the UK's National Health Service (NHS) reported 346,217 new cancer diagnoses in 2022, averaging 948 cases per day. This marks a 5% increase from the 329,664 diagnoses in 2021. Additionally, cancer diagnoses saw a 7% rise, going from 167,917 to 180,877 in 2022. Consequently, the growing prevalence of cancer cases is driving the bevacizumab biosimilars market.
The rising healthcare expenditure is projected to drive the growth of the bevacizumab biosimilars market in the future. Healthcare expenditure refers to the total amount of money spent on healthcare goods and services within a specific timeframe, typically expressed as a monetary value. The bevacizumab biosimilars play a significant role in influencing healthcare expenditure by providing cost-effective alternatives to the originator drug. For example, in 2022, the Office for National Statistics, a UK-based national statistical authority, reported that healthcare expenditure in the UK reached approximately £283 billion. This reflects a 0.7% increase in nominal terms compared to spending in 2021. Thus, the rise in healthcare expenditure is driving the bevacizumab biosimilars market.
Product innovation has become a prominent trend gaining traction in the bevacizumab biosimilars market. Key market players are strategically focusing on the development of inventive products to enhance their market positions. An illustrative example is the collaborative effort of Viatris, a US-based pharmaceutical company, and Biocon Biologics, a US-based pharmaceutical company, in May 2022, resulting in the launch of a biosimilar named Abevmy (bevacizumab). This biosimilar is designed to mirror Roche's Avastin (bevacizumab) and has received approval from Health Canada for application in four oncology indications. Abevmy boasts efficacy, safety, and quality comparable to the reference biologic, as it is a synthesized humanized monoclonal antibody (MA) that binds to and neutralizes the biologic action of human vascular endothelial growth factor (VEGF). This exemplifies the trend of product innovation as a strategic focus within the bevacizumab biosimilars market.
Leading companies in the bevacizumab biosimilars market are strategically engaging in collaborations to develop Next-Generation Biosimilars, a move aimed at fortifying and sustaining their market positions. Next-generation biosimilars contribute to the advancement of the bevacizumab biosimilars market through technological enhancements, improved manufacturing processes, and heightened therapeutic efficacy. A noteworthy example is the exclusive partnership formed in May 2022 between Prestige Biopharma, a Singapore-based pharmaceutical company, and Intas Pharmaceuticals, a multinational pharmaceutical company based in India. This collaboration focuses on Prestige's bevacizumab biosimilar, HD204, currently in Phase III development and designed to replicate Roche's Avastin for various cancers. The exclusive agreement covers key regions such as the US, Europe, and parts of Asia and Africa. With HD204 positioned to offer advancements in bevacizumab biosimilar technology, the collaboration allows Intas Pharmaceuticals to commercialize the biosimilar, leveraging its robust sales and marketing capabilities. This strategic alignment supports Prestige's broader goal of expanding market reach and providing affordable access to patients through its innovative bevacizumab production technology.
In November 2022, Biocon Biologics Ltd., an India-based firm and subsidiary of Biocon Ltd. engaged in the manufacturing of biopharmaceuticals, successfully acquired Viatris Inc.'s global biosimilars business for an undisclosed amount. This strategic acquisition is geared towards reinforcing Biocon Biologics' presence in the biosimilars market. Viatris Inc., based in the US, is a pharmaceutical and healthcare company specializing in medicines for major therapeutic areas, including cancer, with notable products such as Bevacizumab.
Major companies operating in the bevacizumab biosimilars market include Cipla Limited, Reliance lifesciences Pvt. Ltd., Fujifilm Kyowa Kirin Biologics Co. Ltd., Pfizer Inc., F. Hoffmann-La Roche Ltd., Teva Pharmaceutical Industries Ltd., Mylan Inc., Daiichi Sankyo Company Limited, Beaconpharma Ltd., Innovent Biologics Inc., STADA Arzneimittel AG, Shanghai Henlius Biotech Inc., Aurobindo Pharma Ltd., Dr. Reddy's Laboratories Ltd., Biocon Ltd., Intas Pharmaceuticals Ltd., Amneal Pharmaceuticals LLC, Samsung Bioepis Co. Ltd., Celltrion Inc., Coherus BioSciences Inc., Hetero Drugs Ltd., Apotex Inc., BioXpress Therapeutics SA, mAbxience SA.
North America was the largest region in the bevacizumab biosimilars market in 2024. The regions covered in the bevacizumab biosimilars market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the bevacizumab biosimilars market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the resulting trade tensions in spring 2025 are having a significant impact on the pharmaceutical sector. Companies are grappling with higher costs on imported active pharmaceutical ingredients (APIs), glass vials, and laboratory equipment - many of which have limited alternative sources. Generic drug manufacturers, already operating with minimal profit margins, are particularly affected, with some scaling back production of low-margin medications. Biotech firms are also experiencing delays in clinical trials due to shortages of specialized reagents linked to tariffs. In response, the industry is shifting API production to regions like India and Europe, building up inventory reserves, and advocating for tariff exemptions on essential medicines.
The bevacizumab biosimilar market research report is one of a series of new reports that provides bevacizumab biosimilar market statistics, including bevacizumab biosimilar industry global market size, regional shares, competitors with a bevacizumab biosimilar market share, detailed bevacizumab biosimilar market segments, market trends, and opportunities, and any further data you may need to thrive in the bevacizumab biosimilar industry. This bevacizumab biosimilar market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
Bevacizumab biosimilars are a specific class of medications that closely resemble a reference biologic drug named bevacizumab. These biosimilars are designed to mimic the original drug's properties and are frequently utilized to impede the formation of new blood vessels within tumors. By inhibiting this process, they effectively decelerate the progression of tumors.
Bevacizumab biosimilar products, including Avastin, Mvasi, Zirabev, Aybintio, and similar alternatives, represent a range of anti-angiogenic therapies designed to impede new blood vessel formation. These products are accessible through diverse distribution channels such as hospital pharmacies, online platforms, and retail outlets. Their applications span across treating colorectal cancer, non-small cell lung cancer, glioblastoma, renal cell carcinoma, cervical cancer, and ovarian cancer.
The bevacizumab biosimilars market consists of sales of byvasda, cizumab, alymsys, bevax, and cyramza. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 1-3 business days.
Table of Contents
Executive Summary
Bevacizumab Biosimilars Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on bevacizumab biosimilars market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for bevacizumab biosimilars? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The bevacizumab biosimilars market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Product: Avastin; Mvasi; Zirabev; Aybintio; Other Products2) By Distribution Channel: Hospital Pharmacy; Online Pharmacy; Retail Pharmacy; Other Distribution Channels
3) By Application: Colorectal Cancer; Non-small Cell Lung Cancer; Glioblastoma; Renal Cell Carcinoma; Cervical Cancer; Ovarian Cancer
Subsegments:
1) By Avastin: Original Avastin Product2) By Mvasi: Mvasi (Amgen Biosimilar)
3) By Zirabev: Zirabev (Pfizer Biosimilar)
4) By Aybintio: Aybintio (Samsung Bioepis Biosimilar)
5) By Other Products: Additional Bevacizumab Biosimilars
Companies Mentioned: Cipla Limited; Reliance lifesciences Pvt. Ltd.; Fujifilm Kyowa Kirin Biologics Co. Ltd.; Pfizer Inc.; F. Hoffmann-La Roche Ltd.; Teva Pharmaceutical Industries Ltd.; Mylan Inc.; Daiichi Sankyo Company Limited; Beaconpharma Ltd.; Innovent Biologics Inc.; STADA Arzneimittel AG; Shanghai Henlius Biotech Inc.; Aurobindo Pharma Ltd.; Dr. Reddy's Laboratories Ltd.; Biocon Ltd.; Intas Pharmaceuticals Ltd.; Amneal Pharmaceuticals LLC; Samsung Bioepis Co. Ltd.; Celltrion Inc.; Coherus BioSciences Inc.; Hetero Drugs Ltd.; Apotex Inc.; BioXpress Therapeutics SA; mAbxience SA
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Bevacizumab Biosimilars market report include:- Cipla Limited
- Reliance lifesciences Pvt. Ltd.
- Fujifilm Kyowa Kirin Biologics Co. Ltd.
- Pfizer Inc.
- F. Hoffmann-La Roche Ltd.
- Teva Pharmaceutical Industries Ltd.
- Mylan Inc.
- Daiichi Sankyo Company Limited
- Beaconpharma Ltd.
- Innovent Biologics Inc.
- STADA Arzneimittel AG
- Shanghai Henlius Biotech Inc.
- Aurobindo Pharma Ltd.
- Dr. Reddy's Laboratories Ltd.
- Biocon Ltd.
- Intas Pharmaceuticals Ltd.
- Amneal Pharmaceuticals LLC
- Samsung Bioepis Co. Ltd.
- Celltrion Inc.
- Coherus BioSciences Inc.
- Hetero Drugs Ltd.
- Apotex Inc.
- BioXpress Therapeutics SA
- mAbxience SA
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | September 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 1.64 Billion |
| Forecasted Market Value ( USD | $ 2.23 Billion |
| Compound Annual Growth Rate | 7.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |