Global Oncology Clinical Trials Market - Key Trends and Drivers Summarized
Why Are Oncology Clinical Trials Crucial in the Fight Against Cancer?
Oncology clinical trials are at the forefront of cancer research, but why are they so crucial in the fight against this complex disease? Clinical trials in oncology are essential because they are the primary means by which new treatments are tested for safety, efficacy, and overall benefit to patients. These trials pave the way for the development of innovative therapies, including targeted drugs, immunotherapies, and combination treatments that can provide better outcomes than existing options. Given the heterogeneous nature of cancer, with its many subtypes and varied genetic mutations, oncology clinical trials are designed to explore how different treatments work across diverse patient populations. This is particularly important as personalized medicine becomes more prevalent, where treatments are tailored to the individual’ s genetic makeup and specific characteristics of their cancer. The data generated from these trials not only informs clinical practice but also helps shape future research directions. As cancer remains one of the leading causes of death worldwide, the ongoing efforts in clinical trials are vital in finding more effective treatments and ultimately improving survival rates and quality of life for patients.How Do Innovations in Oncology Clinical Trials Impact Patient Care?
The landscape of oncology clinical trials is rapidly evolving, but how do these innovations impact patient care? One of the most significant advancements is the incorporation of precision medicine into trial designs. Precision medicine aims to tailor treatments based on the genetic profiles of individual tumors, and this approach has been integrated into clinical trials to identify which patients are most likely to benefit from specific therapies. This not only improves the likelihood of success in trials but also ensures that patients receive the most effective treatments with fewer side effects. Another innovation is the use of adaptive trial designs, which allow modifications to the trial protocols based on interim results. This flexibility can accelerate the development of promising therapies by allowing researchers to focus resources on the most effective treatments early in the process. Additionally, the rise of decentralized and virtual clinical trials, which utilize digital health technologies and telemedicine, has made it easier for patients to participate in studies, particularly those in remote or underserved areas. This has broadened the reach of oncology trials, ensuring more diverse patient populations are represented. These innovations are transforming how clinical trials are conducted, making them more efficient, patient-centered, and ultimately leading to better outcomes in cancer care.What Are the Latest Trends Shaping the Future of Oncology Clinical Trials?
Oncology clinical trials are being shaped by several key trends, but what are the latest developments that could define their future? One of the most prominent trends is the increasing focus on immuno-oncology, where clinical trials are exploring therapies that harness the immune system to fight cancer. Immunotherapies, such as checkpoint inhibitors and CAR-T cell therapy, have shown remarkable results in certain cancers, leading to a surge in clinical trials testing these approaches in various tumor types. Another significant trend is the growing importance of biomarker-driven trials, which aim to identify biological markers that predict how well a patient will respond to a particular treatment. These trials are crucial in the era of personalized medicine, as they help stratify patients and ensure that those most likely to benefit from a treatment are the ones who receive it. The use of real-world evidence (RWE) in oncology trials is also gaining traction, with data from electronic health records, patient registries, and other sources being used to complement traditional clinical trial data. This approach provides a more comprehensive understanding of how treatments perform in everyday clinical practice. Additionally, the integration of artificial intelligence (AI) and machine learning in clinical trial design and data analysis is streamlining the process, enabling faster and more accurate identification of potential therapies. These trends are driving significant changes in how oncology trials are conducted, with a clear emphasis on precision, efficiency, and patient-centricity.What Factors Are Driving the Growth in the Oncology Clinical Trials Market?
The growth in the oncology clinical trials market is driven by several factors, reflecting the increasing demand for innovative cancer treatments and the evolving landscape of cancer research. One of the primary drivers is the rapid advancement in genomics and molecular biology, which has enabled the development of targeted therapies that require rigorous clinical testing. The rise of personalized medicine, where treatments are tailored to the genetic profile of an individual’ s cancer, has created a need for more specialized and complex trials, contributing to market growth. Another significant factor is the increasing incidence of cancer worldwide, which has led to a greater focus on finding effective treatments, thereby driving the demand for clinical trials. The expansion of global clinical trial networks and collaborations between pharmaceutical companies, research institutions, and healthcare providers has also played a crucial role in facilitating more trials across different regions. Furthermore, the growing acceptance and integration of digital technologies, such as telemedicine and electronic data capture, have streamlined trial processes, making it easier to recruit participants and manage trials remotely. The involvement of patient advocacy groups in trial design and patient recruitment has also enhanced trial participation and ensured that patient needs are prioritized. Together, these factors are fueling the robust growth of the oncology clinical trials market, positioning it as a critical component of the future of cancer treatment.Report Scope
The report analyzes the Oncology Clinical Trials market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Phase Type (Phase I Clinical Trails, Phase II Clinical Trails, Phase III Clinical Trails, Phase IV Clinical Trails); Study Type (Interventional Studies, Observational Studies, Expanded Access Studies).
- Geographic Regions/Countries: World; USA; Canada; Japan; China; Europe; France; Germany; Italy; UK; Spain; Russia; Rest of Europe; Asia-Pacific; Australia; India; South Korea; Rest of Asia-Pacific; Latin America; Argentina; Brazil; Mexico; Rest of Latin America; Middle East; Iran; Israel; Saudi Arabia; UAE; Rest of Middle East; Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Phase I Clinical Trails segment, which is expected to reach US$7.9 Billion by 2030 with a CAGR of a 5.8%. The Phase II Clinical Trials segment is also set to grow at 4.4% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $3.2 Billion in 2024, and China, forecasted to grow at an impressive 8.2% CAGR to reach $3.3 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Oncology Clinical Trials Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Oncology Clinical Trials Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Oncology Clinical Trials Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AbbVie, Inc., AstraZeneca PLC, Caidya™, Eli Lilly and Company, F. Hoffmann-La Roche Ltd. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 43 companies featured in this Oncology Clinical Trials market report include:
- AbbVie, Inc.
- AstraZeneca PLC
- Caidya™
- Eli Lilly and Company
- F. Hoffmann-La Roche Ltd.
- GlaxoSmithKline plc.
- ICON plc
- Medpace, Inc.
- Merck & Co., Inc.
- Novartis AG
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie, Inc.
- AstraZeneca PLC
- Caidya™
- Eli Lilly and Company
- F. Hoffmann-La Roche Ltd.
- GlaxoSmithKline plc.
- ICON plc
- Medpace, Inc.
- Merck & Co., Inc.
- Novartis AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 283 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
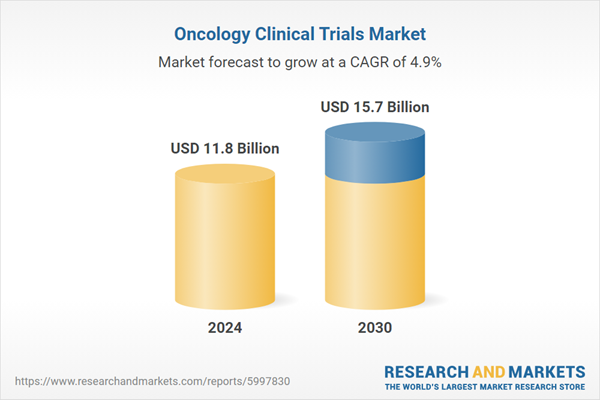
| Estimated Market Value ( USD | $ 11.8 Billion |
| Forecasted Market Value ( USD | $ 15.7 Billion |
| Compound Annual Growth Rate | 4.9% |
| Regions Covered | Global |