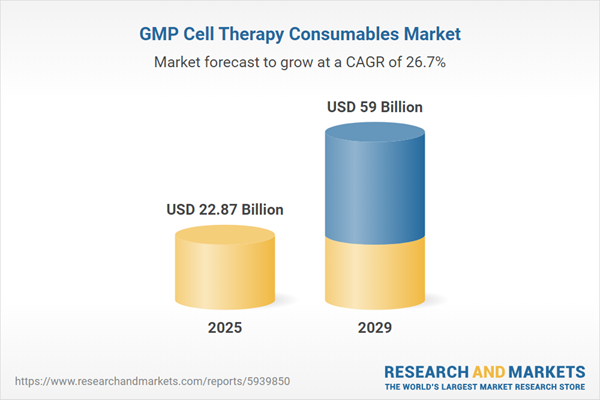
The GMP cell therapy consumables market size is expected to see exponential growth in the next few years. It will grow to $59 billion in 2029 at a compound annual growth rate (CAGR) of 26.7%. The growth in the forecast period can be attributed to expansion of cell therapy applications, global increase in cell therapy trials, regulatory support for cell therapies, emergence of advanced cell therapy platforms, globalization of cell therapy manufacturing. Major trends in the forecast period include investments in manufacturing infrastructure, technological innovations in cell processing, collaborations and partnerships, digitalization and data integration, strategic alliances and mergers.
The growth of the GMP cell therapy consumables market is anticipated to be propelled by the increasing focus on drug discovery. Drug discovery involves the identification of potential new medicinal entities through the integration of computational, experimental, translational, and clinical models. Technological advancements in molecular biology, genetics, bioinformatics, and high-throughput screening have significantly enhanced the drug discovery process. These advancements empower scientists to gain a better understanding of disease mechanisms, identify potential drug targets, and efficiently screen large compound libraries for potential drug candidates. Notably, the Food and Drug Administration (FDA) reported the approval of 37 new drugs and therapeutic biological products by its Center for Drug Evaluation and Research (CDER) in the US in 2022 for various indications, highlighting the surge in drug discovery and its impact on the GMP cell therapy consumables market.
The increasing prevalence of chronic diseases is expected to drive the growth of the GMP cell therapy consumables market. Chronic diseases, characterized by lasting three months or more and potentially worsening over time, have led to intensified research and development efforts in innovative cell therapies to address these persistent conditions. The expansion of cell therapy applications across various chronic diseases has resulted in a heightened demand for GMP consumables to ensure the quality of clinical trials. Stringent regulatory compliance and the growing demand for innovative therapies, driven by the rising number of individuals with chronic diseases (projected to reach 142.66 million worldwide by 2050 according to the National Center for Biotechnology Information), further contribute to the market's growth. In summary, the increasing prevalence of chronic diseases is a significant driver of the GMP cell therapy consumables market.
Leading companies in the GMP cell therapy consumables market are focusing on the development of GMP-compliant CD34+ hematopoietic stem cells (HSCs) to enhance advanced therapies. These GMP-compliant CD34+ HSCs are a specific type of stem cell that expresses the CD34 marker on their surface and is produced following Good Manufacturing Practice (GMP) standards. For example, in April 2024, OrganaBio, a US-based pharmaceutical firm, introduced HematoPAC-HSC-CB-GMP, a readily available source of GMP-compliant CD34+ HSCs obtained from fresh human cord blood. Leveraging its extensive expertise in cell isolation and GMP manufacturing, OrganaBio is able to yield high quantities of highly viable CD34+ HSCs within 24 hours post-collection. This launch aims to support the advancement of next-generation therapies for blood cancers and genetic disorders.
Major companies in the GMP cell therapy consumables market are also concentrating on the development of cutting-edge products, such as cell isolation systems, to gain a competitive advantage. A cell isolation system is a technological platform or device designed to separate specific cells from a mixture based on criteria such as size, markers, or other characteristics. In January 2022, Sony Group Corporation, a Japan-based conglomerate, launched the CGX10 Cell Isolation System, featuring high-speed, high-purity cell sorting within a closed system. The closed structure ensures sterility, making it particularly suitable for applications such as cell-based immunotherapy, where maintaining a sterile environment is crucial. The system, equipped with up to ten parameters and four lasers, enables the isolation of cells with approximately 97% purity at a speed of 15,000 cells per second. The use of a single-use disposable tubing kit minimizes the risk of cross-contamination between samples, showcasing Sony's commitment to advancing cell therapy development and manufacturing.
In September 2022, Cellistic, a Belgium-based company specializing in cell therapies based on human induced pluripotent stem cell (iPSC) technology, acquired Celyad Oncology's GMP-grade cell therapy manufacturing facility for $7.46 million. This strategic acquisition positions Cellistic to leverage the acquired skills and assets, advancing the development of its unique platforms and enhancing the capacity to assist partners in delivering iPSC-based allogeneic cell therapies to patients more efficiently. Celyad Oncology, a renowned biotechnology firm based in Belgium, is dedicated to exploring and developing innovative chimeric antigen receptor (CAR) T-cell therapies designed specifically to combat cancer.
Major companies operating in the GMP cell therapy consumables market include Thermo Fisher Scientific Inc., Fresenius Kabi AG, Danaher Corporation, Merck KGaA, Asahi Kasei Corporation, GE HealthCare Technologies Inc., Corning Incorporated, Avantor Inc., Lonza Group AG, Terumo Corporation, Catalent Inc., Sartorius AG, Bio-Techne Corp, Repligen Corporation, Miltenyi Biotec BV & Co KG, Genscript Biotech Corporation, Rentschler Biopharma SE, FUJIFILM Irvine Scientific Inc., BioLegend Inc., STEMCELL Technologies Inc., BioLife Solutions Inc., Abzena Ltd., Sino Biological Inc., ProBioGen AG, MaxCyte Inc., PromoCell GmbH, PeproTech Inc., Cellares Corp., Wilson Wolf Corporation, Cellexus Ltd.
North America was the largest region in the GMP cell therapy consumables market in 2024. Asia-pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the gmp cell therapy consumables market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the gmp cell therapy consumables market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
GMP cell therapy consumables encompass consumable products and items manufactured in accordance with good manufacturing practices (GMP) guidelines for cell-based therapies. These consumables and tools designed for cell culture assist researchers in cultivating new cells in culture media, catering to various applications such as gene therapy, vaccine production, tissue culture, toxicity testing, and drug development.
The primary categories of GMP cell therapy consumables include kits, reagents or molecular biology reagents, growth factors or cytokines and interleukins, and other essential components. A kit is a compilation of items, such as tools or equipment, intended for a specific activity or purpose. In the realm of GMP cell therapy consumables, kits are utilized for tasks such as the isolation, expansion, harvesting, and formulation of cell therapies. These therapies encompass NK cell therapy, stem cell therapy, T-cell therapy, and others, involving processes such as cell collection and characterization or sorting and separation, cell culture and expansion or preparation, cryopreservation, cell processing and formulation, cell isolation and activation, cell distribution or handling, process monitoring and control or re-administration or quality assurance, among others. GMP cell therapy consumables find application in clinical, commercial, and research settings.
The GMP cell therapy consumables market research report is one of a series of new reports that provides GMP cell therapy consumables market statistics, including the GMP cell therapy consumables industry's global market size, regional shares, competitors with a GMP cell therapy consumables market share, detailed GMP cell therapy consumables market segments, market trends, and opportunities, and any further data you may need to thrive in the GMP cell therapy consumables industry. This GMP cell therapy consumables market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
The GMP cell therapy consumables market consists of sales of cell culture media, media supplements, extracellular matrices, flasks, tubes, dishes or cryovials, serological pipettes, or centrifugation tubes. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
GMP Cell Therapy Consumables Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on gmp cell therapy consumables market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for gmp cell therapy consumables? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The gmp cell therapy consumables market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Product: Kits; Reagents or Molecular Biology reagents; Growth Factors or Cytokines and Interleukins; Other Products2) By Cell Therapy: NK Cell Therapy; Stem Cell Therapy; T-Cell Therapy; Other cell therapies
3) By Process: Cell Collection and Characterization or Sorting and Separation; Cell Culture and Expansion or Preparation; Cryopreservation; Cell Processing and Formulation; Cell Isolation and Activation; Cell Distribution or Handling; Process Monitoring and Control or Readministration or Quality Assurance; Other Processes
4) By End-Use: Clinical; Commercial; Research
Subsegments:
1) By Kits: Cell Culture Kits; Cell Isolation Kits; Transfection Kits2) By Reagents or Molecular Biology Reagents: Buffers and Solutions; Enzymes; Staining Reagents
3) By Growth Factors or Cytokines and Interleukins: Hematopoietic Growth Factors; Antibody Cytokines; Immunomodulatory Cytokines
3) By Other Products: Cell Culture Vessels; Bioreactors; Media and Supplements
Key Companies Mentioned: Thermo Fisher Scientific Inc.; Fresenius Kabi AG; Danaher Corporation; Merck KGaA; Asahi Kasei Corporation
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
- Thermo Fisher Scientific Inc.
- Fresenius Kabi AG
- Danaher Corporation
- Merck KGaA
- Asahi Kasei Corporation
- GE HealthCare Technologies Inc.
- Corning Incorporated
- Avantor Inc.
- Lonza Group AG
- Terumo Corporation
- Catalent Inc.
- Sartorius AG
- Bio-Techne Corp
- Repligen Corporation
- Miltenyi Biotec BV & Co KG
- Genscript Biotech Corporation
- Rentschler Biopharma SE
- FUJIFILM Irvine Scientific Inc.
- BioLegend Inc.
- STEMCELL Technologies Inc.
- BioLife Solutions Inc.
- Abzena Ltd.
- Sino Biological Inc.
- ProBioGen AG
- MaxCyte Inc.
- PromoCell GmbH
- PeproTech Inc.
- Cellares Corp.
- Wilson Wolf Corporation
- Cellexus Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | February 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 22.87 Billion |
| Forecasted Market Value ( USD | $ 59 Billion |
| Compound Annual Growth Rate | 26.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 30 |