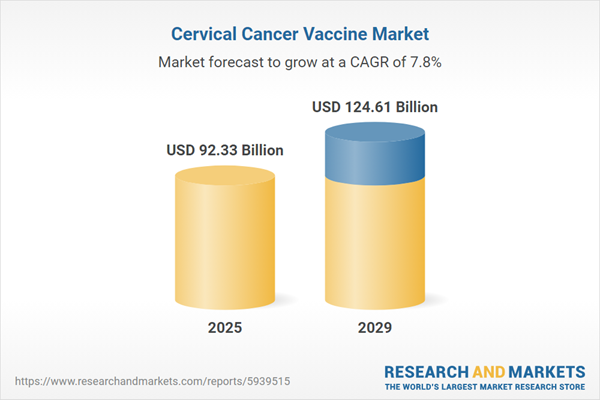
The cervical cancer vaccine market size is expected to see strong growth in the next few years. It will grow to $124.61 billion in 2029 at a compound annual growth rate (CAGR) of 7.8%. The growth in the forecast period can be attributed to expansion of vaccination coverage, integration into routine immunization programs, advancements in hpv testing and screening, global advocacy for hpv vaccination, rising focus on adolescent vaccination. Major trends in the forecast period include incorporation of new adjuvants, integration of digital health platforms, innovative vaccine development, public-private partnerships, educational campaigns and advocacy.
The forecast of 7.8% growth over the next five years reflects a modest reduction of 0.2% from the previous estimate for this market. This reduction is primarily due to the impact of tariffs between the US and other countries. Tariff barriers are expected to hamper U.S. preventive medicine initiatives by increasing the cost of cervical cancer vaccine components imported from Australia and Canada, thereby reducing vaccination rates and elevating long-term cancer treatment expenditures. The effect will also be felt more widely due to reciprocal tariffs and the negative effect on the global economy and trade due to increased trade tensions and restrictions.
The growth trajectory of the cervical cancer vaccine market is anticipated to surge, propelled by the escalating prevalence of cervical cancer cases. Cervical cancer, affecting the lower part of the uterus known as the cervix, is witnessing an increase in reported cases, primarily attributed to factors such as inadequate Human papillomavirus (HPV) vaccination, poor hygiene practices, and limited awareness and knowledge concerning cervical cancer. Notably, cervical cancer vaccines play a pivotal role in preventing this disease by targeting high-risk types of human papillomavirus (HPV). For example, in January 2023, the American Cancer Society reported 13,960 cases of cervical cancer in the United States alone. Consequently, the rising incidence of cervical cancer cases serves as a driving force behind the growth of the cervical cancer vaccine market.
The increase in healthcare spending is significantly driving the growth of the cervical cancer vaccine market. Healthcare spending, which refers to the total financial resources allocated to the healthcare sector in a particular region or country, plays a vital role in supporting efforts for cervical cancer vaccination. Investment in cervical cancer vaccines aims to prevent the disease's onset and reduce the economic burden linked to treatment costs. Additionally, this strategic allocation of resources is designed to improve overall public health outcomes and promote long-term cost-effectiveness by minimizing the need for extensive medical interventions. For example, in May 2023, a report from the Office for National Statistics, a UK-based government department, noted that healthcare spending in the UK rose by 5.6% between 2022 and 2023, compared to a growth rate of 0.9% in 2022. In 2023, UK healthcare expenditure reached approximately $317.63 billion (£292 billion). Therefore, the rise in healthcare spending is a key factor driving the expansion of the cervical cancer vaccine market.
Product innovation stands out as a significant and growing trend within the cervical cancer vaccine market, with prominent market players directing their efforts towards the development of pioneering products to reinforce their market positions. An example of this trend occurred in May 2022 when GlaxoSmithKline, a UK-based pharmaceutical company, obtained approval from China's National Medical Products Administration (NMPA) for a two-dose schedule of its vaccine, Cervarix. This vaccine, intended for girls aged nine to fourteen, has been authorized to prevent cervical cancer. This approval marks a significant milestone as Cervarix becomes the first imported HPV vaccine to utilize a two-dose regimen for this specific age group in mainland China.
Major companies engaged in cervical cancer vaccines are strategically focusing on collaboration and partnerships to deliver reliable services to their clientele. These strategic partnerships involve structured alliances between multiple commercial enterprises, typically established through various business agreements or contracts. For instance, in June 2023, Nykode Therapeutics ASA, a Norway-based provider specializing in next-generation vaccines, expanded its collaboration with Roche Holding AG, a Switzerland-based healthcare company, to advance VB10.16, an off-the-shelf therapeutic cancer vaccine. The collaborative effort aims to evaluate VB10.16 in combination with Roche's atezolizumab (Tecentriq), an anti-PD-L1 immunotherapy, in patients diagnosed with advanced cervical cancer. This partnership holds promise in developing a novel and effective treatment option for a type of cancer that has limited treatment alternatives and typically poor prognoses.
In March 2024, Halma PLC, a UK-based technology company, acquired Rovers Medical Devices for €85 million. This acquisition is aimed at enhancing Halma PLC's offerings in the women's health sector, specifically in cervical cancer diagnostics. Rovers Medical Devices is a Netherlands-based manufacturing company that produces the only self-sampling device equipped with built-in features to ensure accurate sample collection and to provide reassurance to women.
Major companies operating in the cervical cancer vaccine market include Pfizer Inc., Johnson & Johnson, Merck & Co Inc., GlaxoSmithKline plc, Advaxis Inc., Immunovaccine Inc., AstraZeneca PLC, EpiVax Inc., ImmunoVaccine Technologies Inc., OncoOne, Xynomic Pharmaceuticals, Vaxart Inc., Genentech Inc., Astellas Pharma Inc., MedImmune LLC, Vaxine Pty Ltd, Inovio Pharmaceuticals, Advaxis Inc., ImmunoGen Inc., Gritstone Oncology, Inc., VBI Vaccines Inc., Genexine Inc., Aikido Pharma Inc., BioNTech SE, CureVac AG, Karyopharm Therapeutics, Epigenomics AG, Imugene Limited, Immunicum AB, Targovax ASA.
North America was the largest region in the cervical cancer vaccine market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the cervical cancer vaccine market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the cervical cancer vaccine market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the resulting trade tensions in spring 2025 are having a significant impact on the pharmaceutical sector. Companies are grappling with higher costs on imported active pharmaceutical ingredients (APIs), glass vials, and laboratory equipment - many of which have limited alternative sources. Generic drug manufacturers, already operating with minimal profit margins, are particularly affected, with some scaling back production of low-margin medications. Biotech firms are also experiencing delays in clinical trials due to shortages of specialized reagents linked to tariffs. In response, the industry is shifting API production to regions like India and Europe, building up inventory reserves, and advocating for tariff exemptions on essential medicines.
The cervical cancer vaccine market research report is one of a series of new reports that provides cervical cancer vaccine market statistics, including the cervical cancer vaccine industry global market size, regional shares, competitors with a cervical cancer vaccine market share, detailed cervical cancer vaccine market segments, market trends, and opportunities, and any further data you may need to thrive in the cervical cancer vaccine industry. This cervical cancer vaccine market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
The cervical cancer vaccine is designed to prevent cervical cancer by targeting high-risk types of human papillomavirus (HPV), the primary cause of most cervical cancer cases. These vaccines stimulate the body's immune response, generating antibodies to recognize and neutralize the HPV virus.
The main cervical cancer vaccines include Cervarix, Gardasil, and Gardasil 9. Cervarix is specifically formulated to protect against certain HPV strains associated with cervical cancer. These vaccines are distributed through various channels, such as hospital pharmacies, retail pharmacies, and online pharmacies, reaching diverse end-users such as hospitals, biotechnology companies, academic and research organizations, among others.
The cervical cancer vaccine market consists of sales of a gardiquimod, V503, silgard, and Human papillomavirus vaccines. Values in this market are ‘factory gate’ values, that is, the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 1-3 business days.
Table of Contents
Executive Summary
Cervical Cancer Vaccine Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on cervical cancer vaccine market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for cervical cancer vaccine? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The cervical cancer vaccine market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Type: Cervarix; Gardasil; Gardasil 92) By Distribution Channels: Hospital Pharmacies; Retail Pharmacies; Online Pharmacies
3) By End-Users: Hospital; Biotechnology Company; Academic and Research Organizations; Other End-Users
Subsegments:
1) By Cervarix: Dosing Schedules; Target Demographics2) By Gardasil: Dosing Schedules; Target Demographics
3) By Gardasil 9: Dosing Schedules; Target Demographics
Companies Mentioned: Pfizer Inc.; Johnson & Johnson; Merck & Co Inc.; GlaxoSmithKline plc; Advaxis Inc.; Immunovaccine Inc.; AstraZeneca PLC; EpiVax Inc.; ImmunoVaccine Technologies Inc.; OncoOne; Xynomic Pharmaceuticals; Vaxart Inc.; Genentech Inc.; Astellas Pharma Inc.; MedImmune LLC; Vaxine Pty Ltd; Inovio Pharmaceuticals; Advaxis Inc.; ImmunoGen Inc.; Gritstone Oncology, Inc.; VBI Vaccines Inc.; Genexine Inc.; Aikido Pharma Inc.; BioNTech SE; CureVac AG; Karyopharm Therapeutics; Epigenomics AG; Imugene Limited; Immunicum AB; Targovax ASA
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Cervical Cancer Vaccine market report include:- Pfizer Inc.
- Johnson & Johnson
- Merck & Co Inc.
- GlaxoSmithKline plc
- Advaxis Inc.
- Immunovaccine Inc.
- AstraZeneca PLC
- EpiVax Inc.
- ImmunoVaccine Technologies Inc.
- OncoOne
- Xynomic Pharmaceuticals
- Vaxart Inc.
- Genentech Inc.
- Astellas Pharma Inc.
- MedImmune LLC
- Vaxine Pty Ltd
- Inovio Pharmaceuticals
- Advaxis Inc.
- ImmunoGen Inc.
- Gritstone Oncology, Inc.
- VBI Vaccines Inc.
- Genexine Inc.
- Aikido Pharma Inc.
- BioNTech SE
- CureVac AG
- Karyopharm Therapeutics
- Epigenomics AG
- Imugene Limited
- Immunicum AB
- Targovax ASA
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | September 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 92.33 Billion |
| Forecasted Market Value ( USD | $ 124.61 Billion |
| Compound Annual Growth Rate | 7.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 31 |