Global Pharmaceutical Dissolution Testing Services Market - Key Trends & Drivers Summarized
What Is Pharmaceutical Dissolution Testing and Why Is It Critical?
Pharmaceutical dissolution testing is a key analytical method used in the pharmaceutical industry to assess the rate and extent to which the active pharmaceutical ingredient (API) in a tablet, capsule, or other dosage form is released into a solution. It is an essential test used to evaluate the bioavailability and efficacy of drugs, ensuring that the medication will be absorbed effectively by the body once ingested. The dissolution test helps determine how a drug breaks down in the digestive system, providing insight into its therapeutic performance. Regulatory agencies like the U.S. FDA and the European Medicines Agency (EMA) require dissolution testing as part of the drug approval process to ensure that the medication behaves as intended in vivo.This testing is critical for pharmaceutical companies, as it provides valuable data for developing new medications, determining batch-to-batch consistency, and ensuring compliance with regulatory standards. Dissolution testing also plays a vital role in quality control throughout the drug development and manufacturing processes. By evaluating how drugs release their ingredients, manufacturers can optimize drug formulations and ensure that medications perform as expected in clinical settings, thus improving patient outcomes. This testing also supports the development of generic drugs by confirming that their dissolution profiles are similar to that of the reference product.
Why Is the Pharmaceutical Dissolution Testing Services Market Expanding?
The pharmaceutical dissolution testing services market is expanding due to several key factors, including the rising demand for drug quality and efficacy, increasing regulatory pressure on pharmaceutical manufacturers, and the growth of the generic drug market. One of the primary drivers is the increasing demand for high-quality medications. As the pharmaceutical industry continues to focus on improving patient outcomes, ensuring that drugs are effectively absorbed and deliver the desired therapeutic effect is more important than ever. Dissolution testing is a fundamental part of drug development, allowing pharmaceutical companies to verify that their products meet the necessary standards for bioavailability, which has driven the demand for these services.Regulatory pressure is another key factor contributing to market growth. Regulatory bodies worldwide require pharmaceutical companies to perform dissolution testing to comply with Good Manufacturing Practices (GMP) and other quality control regulations. These regulations ensure that drugs are consistent, safe, and effective. As the global pharmaceutical industry faces increasing scrutiny over product safety, manufacturers are investing more in dissolution testing to meet the stringent standards set by regulatory agencies. This growing demand for compliance has spurred the expansion of dissolution testing services, which are essential for obtaining and maintaining regulatory approval.
The growth of the generic drug market is also playing a significant role in the expansion of the pharmaceutical dissolution testing services market. As generic drugs gain market share due to their lower cost and comparable efficacy to branded products, dissolution testing has become a critical tool for ensuring that generic formulations are bioequivalent to their branded counterparts. This has increased the need for dissolution testing services, as generic manufacturers must demonstrate that their drugs meet the same dissolution profiles as the original drug. With the increasing prevalence of generics in the global healthcare system, the demand for dissolution testing services is expected to rise.
What Key Trends Are Shaping the Future of Pharmaceutical Dissolution Testing Services?
Several key trends are shaping the future of the pharmaceutical dissolution testing services market, including the growing use of automated testing technologies, the rise of in vitro-in vivo correlation (IVIVC), and the increasing demand for rapid and high-throughput testing. One significant trend is the growing adoption of automated dissolution testing technologies. Automation improves the efficiency and consistency of testing by reducing human error and providing more precise measurements. Automated systems can perform dissolution tests with minimal manual intervention, increasing throughput and allowing for real-time data analysis. This technological advancement is making it easier for pharmaceutical companies to perform high-quality dissolution testing on a larger scale, which is driving the demand for these services.The rise of in vitro-in vivo correlation (IVIVC) is another key trend influencing the future of dissolution testing services. IVIVC is a technique that uses dissolution testing data to predict the in vivo behavior of a drug, such as its bioavailability and absorption rate in the human body. As the pharmaceutical industry increasingly moves toward more efficient and cost-effective drug development processes, the use of IVIVC can streamline clinical trials and regulatory approval processes. By linking in vitro dissolution data to in vivo performance, pharmaceutical companies can reduce the need for extensive clinical studies, making drug development faster and less costly. This growing interest in IVIVC is driving the demand for advanced dissolution testing services that can support these sophisticated modeling techniques.
The increasing demand for rapid and high-throughput testing is another important trend shaping the future of the market. As pharmaceutical companies work to speed up the drug development process, there is a growing need for faster, more efficient dissolution testing. High-throughput dissolution testing allows for the simultaneous testing of multiple formulations, helping manufacturers evaluate a wide range of formulations in a shorter time. This trend toward faster testing is pushing the development of more advanced and automated dissolution testing technologies that can provide high-quality results with minimal delays, further driving the growth of the pharmaceutical dissolution testing services market.
What Are the Key Drivers of Growth in the Pharmaceutical Dissolution Testing Services Market?
The growth in the pharmaceutical dissolution testing services market is driven by several factors, including increasing demand for drug development efficiency, technological advancements, and the need for regulatory compliance. One of the key drivers is the growing demand for efficiency in drug development. As pharmaceutical companies aim to shorten time-to-market for new drugs, the need for faster and more accurate dissolution testing has risen. Automation and high-throughput testing allow pharmaceutical companies to accelerate the testing process and move more quickly through the development cycle, contributing to market growth.Technological advancements in dissolution testing equipment and techniques are also driving the market's growth. The development of more advanced dissolution testing systems that incorporate automation, real-time monitoring, and data analytics has improved the accuracy and speed of testing. These innovations are making dissolution testing more accessible, efficient, and cost-effective, which has increased the adoption of these services across pharmaceutical companies. Advanced technologies, such as integrated software platforms for data analysis and testing management, are also making it easier for companies to manage and interpret dissolution data, driving demand for these services.
The increasing focus on regulatory compliance is another key factor contributing to the growth of the pharmaceutical dissolution testing services market. Regulatory agencies require pharmaceutical companies to demonstrate that their drugs meet specific dissolution criteria to ensure safety, efficacy, and quality. With growing global regulatory standards, pharmaceutical companies must invest in dissolution testing services to meet the requirements set by agencies such as the FDA and EMA. Compliance with these regulations is crucial for obtaining and maintaining market approval, and the demand for dissolution testing services will continue to grow as the industry faces stricter regulatory scrutiny.
Lastly, the expansion of the generic drug market is driving the need for dissolution testing services. Generic drugs must demonstrate that they have the same dissolution profile as their branded counterparts to be approved by regulatory authorities. As the demand for generics continues to rise, the need for dissolution testing services to ensure bioequivalence between generic and reference products is also growing. This trend, coupled with the increasing availability of generic drugs worldwide, is expected to significantly contribute to the growth of the dissolution testing services market.
Report Scope
The report analyzes the Pharmaceutical Dissolution Testing Services market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Method (In-Vitro, In-Vivo); Form (Capsules, Tablets, Others); Dissolution Apparatus (Basket, Paddle, Others).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the In-Vitro Method segment, which is expected to reach US$786.9 Million by 2030 with a CAGR of a 5.5%. The In-Vivo Method segment is also set to grow at 8.8% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $210.5 Million in 2024, and China, forecasted to grow at an impressive 6.4% CAGR to reach $188.6 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Pharmaceutical Dissolution Testing Services Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Pharmaceutical Dissolution Testing Services Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Pharmaceutical Dissolution Testing Services Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Agilent Technologies, Inc., Almac Group, BA Sciences, Catalent Pharma Solutions, Charles River Laboratories and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 36 companies featured in this Pharmaceutical Dissolution Testing Services market report include:
- Agilent Technologies, Inc.
- Almac Group
- BA Sciences
- Catalent Pharma Solutions
- Charles River Laboratories
- Copley Scientific
- CoreRx, Inc
- Dalton Pharma Services
- Distek, Inc.
- EAG Laboratories
- Eurofins Scientific
- Intertek Group plc
- Lubrizol Life Science Health
- Pace Analytical Life Sciences
- Reading Scientific Services Ltd (RSSL)
- SOTAX Pharma Services
- Sterling Pharmaceutical Services
- Synergy Bioscience
- Teledyne Hanson Research
- Vici Health Sciences
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Agilent Technologies, Inc.
- Almac Group
- BA Sciences
- Catalent Pharma Solutions
- Charles River Laboratories
- Copley Scientific
- CoreRx, Inc
- Dalton Pharma Services
- Distek, Inc.
- EAG Laboratories
- Eurofins Scientific
- Intertek Group plc
- Lubrizol Life Science Health
- Pace Analytical Life Sciences
- Reading Scientific Services Ltd (RSSL)
- SOTAX Pharma Services
- Sterling Pharmaceutical Services
- Synergy Bioscience
- Teledyne Hanson Research
- Vici Health Sciences
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 166 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
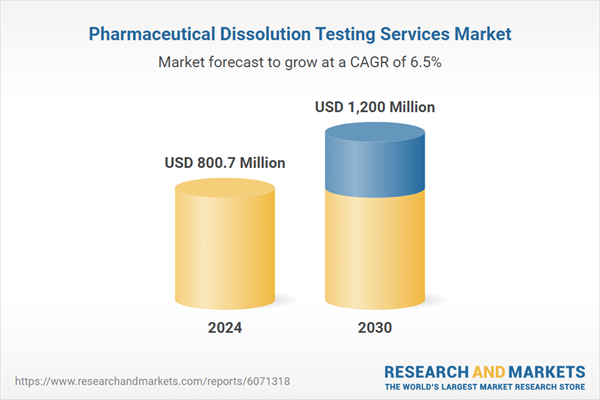
| Estimated Market Value ( USD | $ 800.7 Million |
| Forecasted Market Value ( USD | $ 1200 Million |
| Compound Annual Growth Rate | 6.5% |
| Regions Covered | Global |