The Germany market dominated the Europe Bioimplants Market by Country in 2023, and would continue to be a dominant market till 2031; thereby, achieving a market value of $16.51 billion by 2031. The UK market is exhibiting a CAGR of 6.7% during (2024 - 2031). Additionally, The France market would experience a CAGR of 8.5% during (2024 - 2031).
Increased spending by governments and individuals, particularly in developing countries, has significantly improved access to advanced medical treatments, including these implants. This trend contributes to the overall expansion of the healthcare sector, facilitating the adoption of innovative technologies and improving patient outcomes.
There has been an increase in government expenditure on healthcare, which is being driven by the necessity to address public health challenges and enhance the quality of care. Many countries are increasing their healthcare budgets to expand access to medical services and support the development of advanced treatments. This increase in funding has enabled healthcare systems to invest in cutting-edge medical technologies, including these implants, which are essential for treating various conditions, such as cardiovascular diseases, orthopedic disorders, and dental problems.
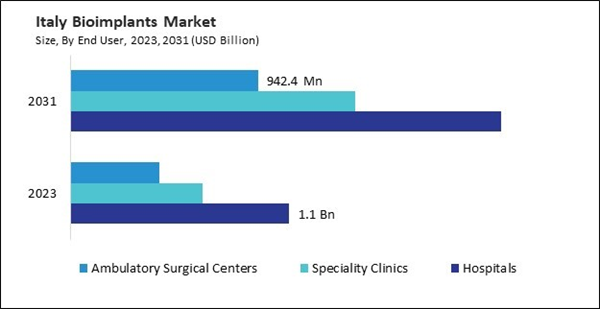
Italy’s healthcare system, Servizio Sanitario Nazionale (SSN), provides favorable reimbursement policies for bioimplant procedures, covering a substantial portion of the costs for medically necessary treatments. These policies make bioimplant surgeries more accessible to the elderly, the primary beneficiaries of such treatments. The aging population and supportive healthcare coverage drive Italy's growing bioimplant demand. Likewise, Spain’s public healthcare system, Sistema Nacional de Salud (SNS), offers extensive reimbursement for various bioimplant procedures, ensuring these treatments are affordable and accessible. This combination of a growing elderly demographic and supportive reimbursement policies drives the demand for these implants in Spain. Therefore, the region will present lucrative growth opportunities for the market.
Based on End User, the market is segmented into Hospitals, Speciality Clinics, and Ambulatory Surgical Centers. Based on Type, the market is segmented into Orthopedic Bioimplants, Cardiovascular Bioimplants, Spinal Bioimplants, Dental Bioimplants, Ophthalmology Bioimplants, and Others. Based on countries, the market is segmented into Germany, UK, France, Russia, Spain, Italy, and Rest of Europe.
List of Key Companies Profiled
- Stryker Corporation
- Arthrex, Inc.
- Zimmer Biomet Holdings, Inc.
- Medtronic PLC
- Smith & Nephew PLC
- Dentsply Sirona Inc.
- Johnson & Johnson
- Boston Scientific Corporation
- Abbott Laboratories
- Victrex plc
Market Report Segmentation
By Price Range (Volume, Thousand Units (30 ml), USD Billion, 2020-2031)- Low
- Mid-range
- Premium Range
- Conventional
- Organic
- Hypermarket & Supermarket
- Online
- Wholesaler & Distributor
- Specialty Stores
- Exclusive Stores
- Others
- Bottled
- Jar
- Other
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
Table of Contents
Companies Mentioned
- Stryker Corporation
- Arthrex, Inc.
- Zimmer Biomet Holdings, Inc.
- Medtronic PLC
- Smith & Nephew PLC
- Dentsply Sirona Inc.
- Johnson & Johnson
- Boston Scientific Corporation
- Abbott Laboratories
- Victrex plc