The Germany market dominated the Europe Bioprocess Validation Market by country in 2024, and is expected to continue to be a dominant market till 2032; thereby, achieving a market value of USD 57.4 million by 2032. The UK market is exhibiting a CAGR of 7.3% during 2025-2032. Additionally, the France market is expected to experience a CAGR of 9.1% during 2025-2032. The Germany and UK led the Europe Bioprocess Validation Market by Country with a market share of 24% and 16.9% in 2024.
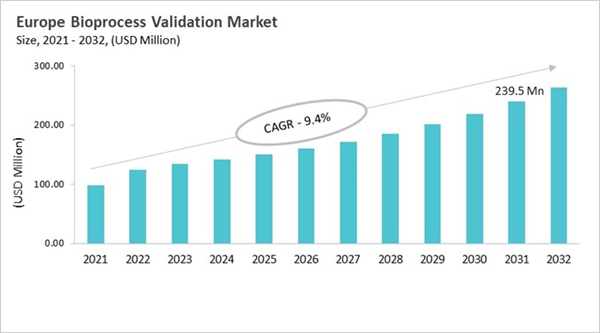
The bioprocess validation market in Europe has grown from simple equipment checks to a full-fledged discipline that follows strict EMA guidelines and is in line with ICH and GMP standards throughout the product's life cycle. Early validation focused on cleaning and testing the final product for small biologics. Now, however, modern methods include process design, risk assessment, and continuous verification. Adopting new technologies like single-use systems, modular facilities, faster processing, and process analytical technologies (PAT) requires complicated validation for extractables, leachables, and system integrity. Digital monitoring and real-time analytics make it possible to control things ahead of time and get products out faster. Biotech clusters in Germany, the UK, France, Switzerland, and the Nordics all work together to create a healthy ecosystem. Contract development and manufacturing organizations (CDMOs) have taken on more responsibilities by providing specialized validation services to meet a wide range of regulatory and manufacturing needs.
Some important trends in the market are real-time, model-based validation, a lot of rules for single-use and modular systems, and more work being sent to specialized providers. Leading companies use strategies like putting hardware and validation services together, putting money into digital tools and predictive analytics, and keeping a strong presence in each region to follow local rules. Partnerships, acquisitions, and lean, risk-based validation methods make a business more competitive. Thought leadership and regulatory engagement, on the other hand, make a business more credible. There is competition among global OEMs that offer integrated solutions, independent validation specialists, and local firms. In a market where audit readiness and regulatory trust define success, innovation in automation and digital platforms is becoming an important way to stand out. A good reputation for compliance is still important.
Stage Outlook
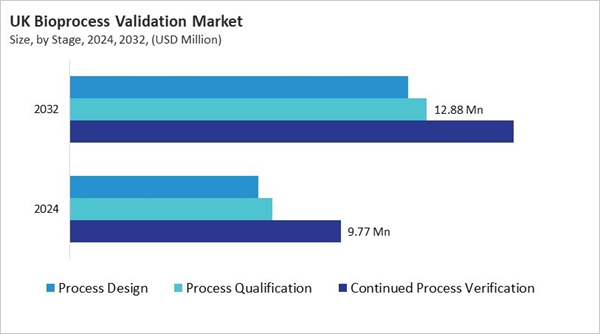
Based on Stage, the market is segmented into Continued Process Verification, Process Qualification, and Process Design. The Continued Process Verification market segment dominated the UK Bioprocess Validation Market by Stage is expected to grow at a CAGR of 6.6 % during the forecast period thereby continuing its dominance until 2032. Also, the Process Design market is anticipated to grow as a CAGR of 7.8 % during the forecast period during 2025-2032.Testing Type Outlook
Based on Testing Type, the market is segmented into Bioprocess Residuals Testing, Extractables & Leachables Testing, Viral Clearance Testing, Wireless and IoT Penetration Testing, and Other Testing Type. Among various Germany Bioprocess Validation Market by Testing Type; The Bioprocess Residuals Testing market achieved a market size of USD $9.5 Million in 2024 and is expected to grow at a CAGR of 6.1 % during the forecast period. The Viral Clearance Testing market is predicted to experience a CAGR of 7.7% throughout the forecast period from (2025 - 2032).Country Outlook
Germany is a major European center for bioprocess validation because it has a strong biotech and pharmaceutical base, advanced research infrastructure, and strict regulations from the EMA. Big companies like Bayer, Boehringer Ingelheim, BioNTech, Merck KGaA, and Sartorius work with specialized CROs to make sure that process design, qualification, and ongoing verification are all done correctly. IoT-enabled bioreactors, real-time digital monitoring, and predictive analytics are some of the Industry 4.0 technologies that are changing validation by automating it and using data to control it. As more money goes into biologics, cell, and gene therapies, the need for more advanced analytical methods, like viral clearance and extractables testing, grows. More and more small and medium-sized businesses are hiring CROs to do validation work because they have specialized knowledge and can do it faster. Competition isList of Key Companies Profiled
- Merck KGaA
- Thermo Fisher Scientific, Inc.
- SGS S.A.
- Eurofins Scientific SE
- Sartorius AG
- Charles River Laboratories International, Inc.
- Lonza Group Ltd.
- WuXi AppTec Co., Ltd.
- Danaher Corporation
- Cobetter Filtration equipment Co., Ltd.
Market Report Segmentation
By Mode
- In house
- Outsourced
By Stage
- Continued Process Verification
- Process Qualification
- Process Design
By Testing Type
- Bioprocess Residuals Testing
- Extractables & Leachables Testing
- Viral Clearance Testing
- Wireless and IoT Penetration Testing
- Other Testing Type
By Country
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
Table of Contents
Companies Mentioned
- Merck KGaA
- Thermo Fisher Scientific, Inc.
- SGS S.A.
- Eurofins Scientific SE
- Sartorius AG
- Charles River Laboratories International, Inc.
- Lonza Group Ltd.
- WuXi AppTec Co., Ltd.
- Danaher Corporation
- Cobetter Filtration equipment Co., Ltd.