The Germany market dominated the Europe Sternal Closure Systems Market by Country in 2023, and would continue to be a dominant market till 2031; thereby, achieving a market value of $230.6 million by 2031. The UK market is exhibiting a CAGR of 3.9% during (2024 - 2031). Additionally, The France market would experience a CAGR of 5.6% during (2024 - 2031).
The rising prevalence of orthaveaedic conditions such as fractures, spinal deformities, and degenerative joint diseases has led to a significant increase in the number of orthopaedic surgeries. This increased surgical volume directly drives the demand for it.
Additionally, Spinal fusion surgeries are becoming increasingly common for the treatment of spinal disorders like degenerative disc disease, spinal stenosis, and spinal fractures. Stabilizing the sternum effectively with advanced sternal closure systems is essential for ensuring successful outcomes in these procedures.
Ischemic heart disorders, including myocardial infarction (MI), are significant health concerns in France. The increasing incidence of these conditions drives the demand for cardiac surgeries, including CABG, which requires sternal closure systems in France. France is known for its advancements in cardiovascular medicine and surgery. The country's healthcare system prioritizes innovation and innovative technology, such as sternal closure devices, to enhance patient outcomes and lower the risk of complications.
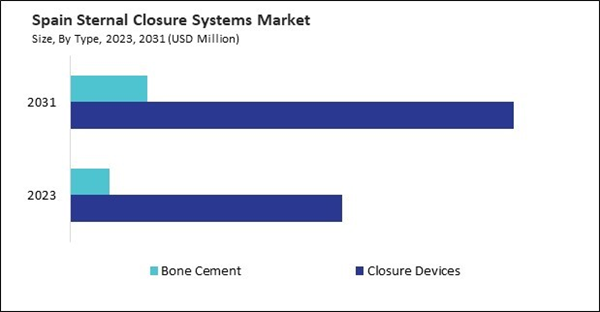
Based on Type, the market is segmented into Closure Devices and Bone Cement. Based on Procedure, the market is segmented into Median Sternotomy, Hemisternotomy, Bilateral Thoracosternotomy, and Others. Based on Material Type, the market is segmented into Titanium, Stainless Steel, Polyether Ether Ketone (PEEK) and Others. Based on countries, the market is segmented into Germany, UK, France, Russia, Spain, Italy, and Rest of Europe.
List of Key Companies Profiled
- Johnson & Johnson
- KLS Martin Group
- Orthofix Medical, Inc. (Breg, Inc.)
- Kinamed Inc.
- Braun Melsungen AG
- Stryker Corporation
- Medtronic PLC
- Zimmer Biomet Holdings, Inc.
- Acumed LLC (Colson Medical, Inc.)
- Able Medical Devices
Market Report Segmentation
By Type
- Closure Devices
- Bone Cement
By Procedure
- Median Sternotomy
- Hemisternotomy
- Bilateral Thoracosternotomy
- Others
By Material Type
- Titanium
- Stainless Steel
- Polyether Ether Ketone (PEEK)
- Others
By Country
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
Table of Contents
Companies Mentioned
- Johnson & Johnson
- KLS Martin Group
- Orthofix Medical, Inc. (Breg, Inc.)
- Kinamed Inc.
- B. Braun Melsungen AG
- Stryker Corporation
- Medtronic PLC
- Zimmer Biomet Holdings, Inc.
- Acumed LLC (Colson Medical, Inc.)
- Able Medical Devices