Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
The market is characterized by the use of flow diversion devices, a minimally invasive treatment designed to reroute blood flow away from aneurysms, aiding in their occlusion and reducing the risk of rupture. Continuous advancements in flow diversion technologies and neuroimaging techniques are broadening treatment options and enhancing patient outcomes. The rising incidence of cerebral aneurysms, often linked to aging and other risk factors, is further propelling demand for effective treatment solutions. Compared to traditional surgical approaches, flow diversion procedures are less invasive, leading to shorter recovery times and reduced hospital stays.
Key Market Drivers
The Flow Diversion Aneurysm Treatment Market is experiencing notable growth, primarily driven by the increasing adoption of minimally invasive treatment options. This trend reflects a shift in the management of cerebral aneurysms, prioritizing less invasive procedures over traditional open surgeries. Several factors contributing to this shift include:Minimally invasive procedures typically require smaller incisions or access points, resulting in reduced tissue damage, less pain, and faster recovery times compared to open surgery. This approach is highly appealing to both patients and healthcare providers.
Flow diversion treatments yield excellent patient outcomes, with lower complication rates and reduced risks of infection or bleeding, which boosts patient satisfaction and enhances the reputation of these procedures.
Advances in medical devices and imaging technologies have made minimally invasive treatments more precise and effective. Real-time imaging guidance plays a critical role in increasing the accuracy of flow diversion procedures.
Key Market Challenges
Despite the promising growth of the market, high procedural costs remain a significant challenge. These costs encompass various aspects of treatment, including the price of flow diversion devices, hospital fees, physician charges, and post-procedure care. Contributing factors to the elevated costs include:Flow diversion devices, while highly effective, can be expensive, and the cost of these specialized devices forms a substantial part of the overall treatment expenses.
Performing flow diversion procedures requires specialized training, which incurs additional costs for education and certification.
The complexity of pre-operative assessments, extended hospital stays, and follow-up care also increases overall healthcare costs.
While technological advancements in devices and medical tools may lead to better outcomes, they can also result in higher upfront costs.
Reimbursement rates for flow diversion procedures in some healthcare systems may not fully cover the costs, creating financial constraints for hospitals and healthcare providers. High procedural costs can also limit patient access to these advanced treatments.
Addressing these challenges requires a multi-pronged approach, including optimizing the cost-effectiveness of flow diversion devices, improving reimbursement strategies, and expanding access to specialized care. Overcoming these barriers is essential to ensuring equitable patient access to life-saving treatments and ensuring the sustainability of healthcare systems.
Key Market Trends
A growing adoption of flow diversion techniques is a prominent trend in the market, with an increasing preference for flow diversion procedures as the primary method for managing cerebral aneurysms. Key factors contributing to this trend include:Flow diversion procedures are less invasive compared to traditional open surgeries, attracting patients seeking treatments that offer quicker recovery times, reduced pain, and smaller incisions.
Flow diversion techniques have proven to be highly effective in treating complex aneurysms, such as wide-necked and fusiform aneurysms, that were previously difficult to manage. This effectiveness has led to greater trust and acceptance among both physicians and patients.
Ongoing innovations in flow diversion devices, including improvements in design, materials, and delivery systems, have enhanced their safety and efficacy, further promoting adoption.
Patients undergoing flow diversion procedures often experience positive outcomes, including lower rates of aneurysm recurrence and fewer complications, contributing to higher patient satisfaction and confidence in the treatment.
An increasing number of neurointerventionalists are being trained in flow diversion techniques, expanding the availability of these procedures in medical centers globally. The growing body of clinical evidence supporting the effectiveness of flow diversion in preventing aneurysm rupture is further driving its adoption.
Key Market Players
- Acandis GmbH
- BALT Group
- Cardinal Health Inc
- Endologix LLC
- Evasc Medical Systems Corp
- Imperative Care Inc
- InspireMD Inc
- Johnson & Johnson
- Lepu Medical Technology Beijing Co. Ltd
- Medtronic Plc
Market Segmentation
This report segments the global Flow Diversion Aneurysm Treatment Market into the following categories:
By Product:
- Flow Diverter Stents (FDS)
- Intrasaccular Flow Disruption Devices
By End User:
- Hospitals
- Specialty Clinics
By Region:
- North America (United States, Canada, Mexico)
- Europe (France, United Kingdom, Italy, Germany, Spain)
- Asia-Pacific (China, India, Japan, Australia, South Korea)
- South America (Brazil, Argentina, Colombia)
- Middle East & Africa (South Africa, Saudi Arabia, UAE)
Competitive Landscape
The report provides a detailed analysis of the major players in the Flow Diversion Aneurysm Treatment Market, including their strategies, market share, and key business developments.Available Customizations
TechSci Research offers customizations for this global Flow Diversion Aneurysm Treatment Market report according to specific company needs. Customization options include:
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Acandis GmbH
- BALT Group
- Cardinal Health Inc
- Endologix LLC
- Evasc Medical Systems Corp
- Imperative Care Inc
- InspireMD Inc
- Johnson and Johnson
- Lepu Medical Technology Beijing Co. Ltd
- Medtronic Plc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | March 2025 |
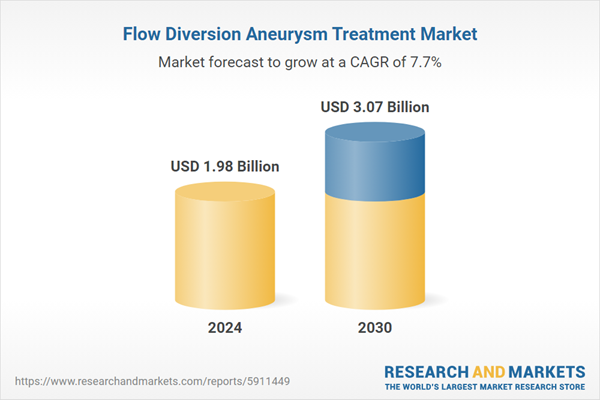
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 1.98 Billion |
| Forecasted Market Value ( USD | $ 3.07 Billion |
| Compound Annual Growth Rate | 7.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |