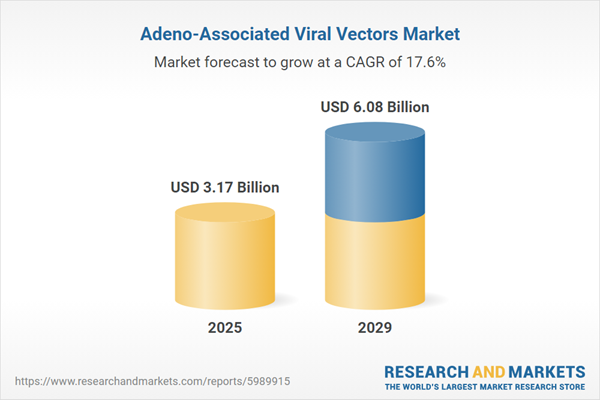
The adeno-associated viral vectors market size has grown rapidly in recent years. It will grow from $2.69 billion in 2024 to $3.17 billion in 2025 at a compound annual growth rate (CAGR) of 17.9%. The growth in the historic period can be attributed to vaccine development, increased funding and investment, academic and industrial collaborations, expansion of clinical trials, and public awareness and advocacy.
The adeno-associated viral vectors market size is expected to see rapid growth in the next few years. It will grow to $6.08 billion in 2029 at a compound annual growth rate (CAGR) of 17.6%. The growth in the forecast period can be attributed to expanding therapeutic applications, increasing the prevalence of genetic disorders, and neurological disorders, regulatory approvals and support, and cost reductions in production. Major trends in the forecast period include gene therapy advancements, technological innovations, scalable manufacturing processes, collaborations and investments, and technological advancements.
The adeno-associated viral vectors market is poised for growth due to the increasing prevalence of genetic disorders. Genetic disorders arise from abnormalities in an individual's DNA, leading to physical or developmental issues. Factors such as improved diagnostic techniques, greater awareness, advanced reproductive age, environmental influences, and genetic drift contribute to the rising prevalence of genetic disorders. Adeno-associated viral vectors play a crucial role in gene therapy for these disorders by delivering corrective genes into target cells, potentially treating conditions such as muscular dystrophy or cystic fibrosis. For example, the World Health Organization (WHO) reported in February 2023 that congenital diseases caused approximately 240,000 infant deaths globally within 28 days of birth annually, with an additional 170,000 deaths in children aged 1 month to 5 years, highlighting the driving force behind the adeno-associated viral vectors market.
Key players in the adeno-associated viral vectors market are emphasizing off-the-shelf availability of replication-capsid plasmids to enhance their competitiveness. Rep/Cap plasmids, widely used in gene therapy for adeno-associated virus (AAV) vector production, are readily accessible from multiple commercial suppliers catering to molecular biology research requirements. For instance, Charles River Laboratories International Inc. introduced an off-the-shelf replication-capsid plasmid range in January 2024, streamlining AAV-based gene therapy initiatives. This expansion of their product portfolio complements existing lentiviral packaging and AAV Helper plasmid offerings, reducing manufacturing efforts by up to 66%. These ready-to-use plasmids undergo batch production with detailed documentation, complying with CMC guidelines and accompanied by Certification of Analysis (COA) to facilitate IND and Clinical Trial Application (CTA) submissions.
In May 2024, Merck KGaA, a Germany-based company specializing in pharmaceuticals, biotechnology, and chemical materials, acquired Mirus Bio LLC for $600 million. This acquisition allows Merck KGaA to enhance its life sciences capabilities by incorporating Mirus Bio’s advanced RNA-based research and therapeutic technologies. As a result, Merck KGaA strengthens its position in the expanding genetic engineering and biotechnology markets, further advancing its commitment to developing innovative biopharmaceutical products. Mirus Bio LLC, a US-based company, specializes in adeno-associated viral (AAV) vectors and gene delivery technologies.
Major companies operating in the adeno-associated viral vectors market are Pfizer Inc., Astellas Pharma, Biogen Inc., Charles River Laboratories International Inc., BioMarin Pharmaceutical Inc., Sarepta Therapeutics Inc., PTC Therapeutics, Ultragenyx Pharmaceutical, Amicus Therapeutics Inc., Oxford Biomedica, Asklepios BioPharmaceutical Inc., uniQure biopharma B.V., Spark Therapeutics Inc., Akouos inc., Adverum Biotechnologies Inc., Passage Bio Inc., AVROBIO Inc, MeiraGTx Holdings plc, GenSight Biologics S.A., Freeline Therapeutics, Aspa Therapeutics Inc., Adrenas Therapeutics Inc., 4D Molecular Therapeutics, Abeona Therapeutics Inc., Neurophth Therapeutics.
North America was the largest region in the adeno-associated viral vectors market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the adeno-associated viral vectors market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the adeno-associated viral vectors market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Adeno-associated viral vectors (AAV) are small, non-pathogenic viruses extensively used in gene therapy to deliver therapeutic genes into target cells, offering potential treatments for genetic disorders, cancers, and various diseases. Their safety profile and efficient infection of both dividing and non-dividing cells make them valuable tools in biomedical research and clinical applications.
The main types of AAV therapy are gene augmentation, immunotherapy, and others. Gene augmentation involves introducing a functional gene to replace or supplement a defective or missing gene in a patient's cells. This therapy can be delivered through ex vivo or in vivo methods, targeting therapeutic areas such as genetic disorders, hematological disorders, infectious diseases, metabolic disorders, ophthalmic disorders, muscle disorders, neurological disorders, and more. The scale of operation ranges from preclinical and clinical stages to commercial applications, applied in gene therapy, cell therapy, and vaccines.
The adeno-associated viral vectors market research report is one of a series of new reports that provides adeno-associated viral vectors market statistics, including adeno-associated viral vectors industry global market size, regional shares, competitors with a adeno-associated viral vectors market share, detailed adeno-associated viral vectors market segments, market trends and opportunities, and any further data you may need to thrive in the adeno-associated viral vectors industry. This adeno-associated viral vectors market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The adeno-associated viral vectors market consists of sales of recombinant adeno-associated viral vectors (AAV), serotype-specific adeno-associated viral vectors (AAV), and custom adeno-associated viral vectors (AAV). Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Adeno-Associated Viral Vectors Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on adeno-associated viral vectors market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for adeno-associated viral vectors ? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The adeno-associated viral vectors market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Type of Therapy: Gene Augmentation; Immunotherapy; Other Type of Therapies2) By Type of Gene Delivery Method Used: Ex Vivo; in Vivo
3) By Target Therapeutic Area: Genetic Disorders; Hematological Disorders; Infectious Diseases; Metabolic Disorders; Ophthalmic Disorders; Muscle Disorders; Neurological Disorders; Other Target Therapeutic Areas
4) By Scale of Operation: Preclinical; Clinical; Commercial
5) By Application Area: Gene Therapy; Cell Therapy; Vaccines
Subsegments:
1) By Gene Augmentation: Inherited Genetic Disorders; Muscular Dystrophy; Cystic Fibrosis; Hemophilia2) By Immunotherapy: Cancer Immunotherapy; Viral Infections Immunotherapy; Autoimmune Diseases Immunotherapy
3) By Other Types of Therapies: Gene Editing; RNA Therapy; Antiviral Therapy
Key Companies Mentioned: Pfizer Inc.; Astellas Pharma; Biogen Inc.; Charles River Laboratories International Inc.; BioMarin Pharmaceutical Inc.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The major companies featured in this Adeno-Associated Viral Vectors market report include:- Pfizer Inc.
- Astellas Pharma
- Biogen Inc.
- Charles River Laboratories International Inc.
- BioMarin Pharmaceutical Inc.
- Sarepta Therapeutics Inc.
- PTC Therapeutics
- Ultragenyx Pharmaceutical
- Amicus Therapeutics Inc.
- Oxford Biomedica
- Asklepios BioPharmaceutical Inc.
- uniQure biopharma B.V.
- Spark Therapeutics Inc.
- Akouos inc.
- Adverum Biotechnologies Inc.
- Passage Bio Inc.
- AVROBIO Inc
- MeiraGTx Holdings plc
- GenSight Biologics S.A.
- Freeline Therapeutics
- Aspa Therapeutics Inc.
- Adrenas Therapeutics Inc.
- 4D Molecular Therapeutics
- Abeona Therapeutics Inc.
- Neurophth Therapeutics
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | April 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 3.17 Billion |
| Forecasted Market Value ( USD | $ 6.08 Billion |
| Compound Annual Growth Rate | 17.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |