Cardiac Bio Implant Market Global Report by Type (Pacemaker, ICDs (Implantable Cardioverter Defibrillator), CRT (Cardiac Resynchronization Therapy)), Аррlісаtіоn (Arrhythmias, Myocardial Ischemia, Acute Myocardial Infarction, Heart Failure), End-User (Hospitals, Ambulatory Surgical Centers, Cardiac Catheterization Laboratories, Others), Countries and Company Analysis 2025-2033.
Cardiac Bio Implant Devices Industry Overview
Patients with cardiovascular disorders can benefit from cardiac bioimplants, which are medical devices that replace or support damaged heart components. These implants consist of artificial cardiac pumps, pacemakers, stents, and heart valves. The danger of rejection is reduced by the smooth integration of cardiac bioimplants, which are made of biocompatible materials. Usually, they are used to treat heart failure, arrhythmias, and aortic stenosis. Technological developments have made cardiac bioimplants more effective, long-lasting, and minimally invasive, improving patient outcomes, shortening recovery periods, and providing a better way to treat complicated cardiac disorders.Due to aging populations, poor lifestyles, and genetic factors, cardiovascular problems are becoming more common, which is driving growth in the cardiac bioimplant devices market. Patient outcomes, device durability, and efficiency have all improved as a result of technological developments in implant materials and designs. Better healthcare infrastructure, increased patient knowledge, and the growing use of minimally invasive procedures are further driving demand. Strong reimbursement and regulatory frameworks, as well as rising healthcare costs worldwide, are all driving the cardiac bioimplant devices market's growth.
Growth Drivers for the Cardiac Bio Implant Devices Market
Minimally Invasive Procedures
Due to aging populations, poor lifestyles, and genetic factors, cardiovascular problems are becoming more common, which is driving growth in the cardiac bioimplant devices market. Patient outcomes, device durability, and efficiency have all improved as a result of technological developments in implant materials and designs. Better healthcare infrastructure, increased patient knowledge, and the growing use of minimally invasive procedures are further driving demand. Strong reimbursement and regulatory frameworks, as well as rising healthcare costs worldwide, are all driving the cardiac bioimplant devices market's growth.Rising Cardiovascular Disease Prevalence
An important factor propelling the cardiac bioimplant devices market's expansion is the rising incidence of cardiovascular illness. The need for heart valves, pacemakers, and stents is increasing as cardiac diseases, such as coronary artery disease, become increasingly common. About 20.1 million persons in the United States who are 20 years of age or older suffer from coronary heart disease, with one heart attack every 40 seconds, or 805,000 each year, according to the Centers for Disease Control and Prevention. The need for bioimplants will increase as a result of the aging population and the rising prevalence of cardiac disease. The need for sophisticated bioimplants to treat chronic cardiovascular illnesses will only increase as these ailments become more prevalent, propelling market expansion. To address this increasing health burden, a move toward implants that are more permanent and effective will be essential.Technological Advancements
One of the main factors propelling the market for cardiac bioimplants devices is technological advances. More robust, effective, and biocompatible implants have been created as a result of advancements in materials, design, and manufacturing techniques. These days, bioimplants - like cardiac valves, pacemakers, and stents - are more accurate, smaller, and customized for each patient, which enhances results and lowers risks. The use of these devices has been further boosted by developments in 3D printing, smart technology, and minimally invasive surgical methods. These developments are driving market expansion by improving treatment efficacy and broadening the patient base suitable for cardiac bioimplants.Challenges in the Cardiac Bio Implant Devices Market
High costs
The market for cardiac bioimplants devices is severely hampered by high prices. The high cost of bioimplant devices is a result of the sophisticated technology, materials, and manufacturing techniques used in their creation as well as the specific surgical techniques needed. Patients' access may be restricted as a result, especially in low-income areas or nations with less developed healthcare systems. Furthermore, exorbitant expenses may put a strain on insurers and healthcare providers, preventing the broad use of life-saving cardiac bioimplants in some markets.Regulatory Hurdles
One of the biggest obstacles facing the cardiac bioimplant devices sector is regulatory barriers. Innovation and access to cutting-edge therapies may be slowed by the drawn-out and difficult regulatory procedures for new technologies, which can postpone market introduction. Although necessary, regulatory agencies' strict safety and efficacy standards lengthen the time and expense needed to develop new products. Smaller manufacturers or emerging technologies may be especially impacted by these issues, which could reduce competition and delay the timely delivery of beneficial cardiac bioimplants to patients in need.Cardiac Bio Implant Devices Market Overview by Regions
The market for cardiac bioimplant devices is expanding significantly on a regional basis. Because of its strong research, large prevalence of cardiovascular illnesses, and sophisticated healthcare infrastructures, North America leads the world. Europe is next, where aging populations and new technology are driving up demand. The Asia Pacific region is expanding quickly due to improved medical infrastructure, increased disease rates, and easier access to healthcare. As healthcare investments rise in Latin America and the Middle East, these regions are also growing.United States Cardiac Bio Implant Devices Market
Due to the high frequency of cardiovascular disorders, such as heart failure and coronary heart disease, the US cardiac bioimplant market is a major global leader. Technological developments in bioimplant devices, such as heart valves, pacemakers, and stents, as well as a strong healthcare system, help the market. Impulse Dynamics approved and introduced the Optimizer Smart Mini Implantable Pulse Generator (IPG) in the United States in April 2022. By treating moderate to severe heart failure, this Class III medical device gives patients even more therapy alternatives.The demand for cardiac bioimplants is rising due to a number of factors, including an aging population, an increase in illness rates, and growing patient awareness. Furthermore, the U.S. market is growing and innovating due to advantageous reimbursement rules, better medical infrastructure, and continuous research.
Germany Cardiac Bio Implant Devices Market
Due to a strong healthcare system and a high prevalence of cardiovascular illnesses, Germany has one of the biggest cardiac bioimplant markets in Europe. The market for pacemakers, stents, and cardiac valves is growing as a result of an aging population and rising demand for cutting-edge therapies. The adoption of novel bioimplant technologies is facilitated by Germany's emphasis on medical innovation and research as well as its advantageous reimbursement regulations. Continuous improvements in implant materials and design support expansion and enhance patient outcomes nationwide.India Cardiac Bio Implant Devices Market
The market for cardiac bioimplants is expanding quickly in India because to the rising incidence of cardiovascular diseases (CVDs), which rank among the nation's top causes of mortality. The World Health Organization reports that more than 27% of deaths in India are caused by CVDs. The adoption of cutting-edge medical technologies, increased awareness of cardiac disease, and better healthcare infrastructure all help the industry. Because of government healthcare programs and cost reductions, bioimplants such as heart valves, pacemakers, and stents are becoming more widely available. In the upcoming years, there will likely be a significant increase in demand for cardiac bioimplants due to the growing middle class and the growing medical tourism industry.Saudi Arabia Cardiac Bio Implant Devices Market
The market for cardiac bioimplants in Saudi Arabia is expanding quickly due to the country's high rate of cardiovascular disorders, which are a major cause of death. The need for sophisticated therapies like pacemakers, stents, and cardiac valves is growing as the population ages and the number of lifestyle-related illnesses rises. The market is expanding as a result of government investments in healthcare infrastructure and advancements in medical technology. Additionally, the use of cardiac bioimplants is accelerating throughout Saudi Arabia owing to an emphasis on patient knowledge and healthcare accessibility.Global Cardiac Bio Implant Device Market Segments
Туре1. Pacemaker
2. ICDs (Implantable Cardioverter Defibrillator)
3. CRT (Cardiac Resynchronization Therapy)
Аррlісаtіоn
1. Arrhythmias
2. Myocardial Ischemia
3. Acute Myocardial Infarction
4. Heart Failure
End-User
1. Hospitals2. Ambulatory Surgical Centers
3. Cardiac Catheterization Laboratories
4. Others
Countries
North America
1. United States2. Canada
Europe
1. France2. Germany
3. Italy
4. Spain
5. United Kingdom
6. Belgium
7. Netherlands
8. Turkey
Asia Pacific
1. China2. Japan
3. India
4. South Korea
5. Thailand
6. Malaysia
7. Indonesia
8. Australia
9. New Zealand
Latin America
1. Brazil2. Mexico
3. Argentina
Middle East & Africa
1. Saudi Arabia2. UAE
3. South Africa
Rest of the World
All the Key players have been covered from 5 Viewpoints:
1. Overview2. Key Persons
3. Product Portfolio
4. Recent Development & Strategies
5. Revenue Analysis
Key Players Analysis
1. F. Hoffman-La Roche2. Eurofins Scientific
3. Illumina, Inc.
4. Natera Inc.
5. Abbott Laboratories
6. Thermo Fisher Scientific
7. Quest Diagnostics
8. Agilent Technologies
Table of Contents
Companies Mentioned
- F. Hoffman-La Roche
- Eurofins Scientific
- Illumina, Inc.
- Natera Inc.
- Abbott Laboratories
- Thermo Fisher Scientific
- Quest Diagnostics
- Agilent Technologies
Methodology
In this report, for analyzing the future trends for the studied market during the forecast period, the publisher has incorporated rigorous statistical and econometric methods, further scrutinized by secondary, primary sources and by in-house experts, supported through their extensive data intelligence repository. The market is studied holistically from both demand and supply-side perspectives. This is carried out to analyze both end-user and producer behavior patterns, in the review period, which affects price, demand and consumption trends. As the study demands to analyze the long-term nature of the market, the identification of factors influencing the market is based on the fundamentality of the study market.
Through secondary and primary researches, which largely include interviews with industry participants, reliable statistics, and regional intelligence, are identified and are transformed to quantitative data through data extraction, and further applied for inferential purposes. The publisher's in-house industry experts play an instrumental role in designing analytic tools and models, tailored to the requirements of a particular industry segment. These analytical tools and models sanitize the data & statistics and enhance the accuracy of their recommendations and advice.
Primary Research
The primary purpose of this phase is to extract qualitative information regarding the market from the key industry leaders. The primary research efforts include reaching out to participants through mail, tele-conversations, referrals, professional networks, and face-to-face interactions. The publisher also established professional corporate relations with various companies that allow us greater flexibility for reaching out to industry participants and commentators for interviews and discussions, fulfilling the following functions:
- Validates and improves the data quality and strengthens research proceeds
- Further develop the analyst team’s market understanding and expertise
- Supplies authentic information about market size, share, growth, and forecast
The researcher's primary research interview and discussion panels are typically composed of the most experienced industry members. These participants include, however, are not limited to:
- Chief executives and VPs of leading corporations specific to the industry
- Product and sales managers or country heads; channel partners and top level distributors; banking, investment, and valuation experts
- Key opinion leaders (KOLs)
Secondary Research
The publisher refers to a broad array of industry sources for their secondary research, which typically includes, however, is not limited to:
- Company SEC filings, annual reports, company websites, broker & financial reports, and investor presentations for competitive scenario and shape of the industry
- Patent and regulatory databases for understanding of technical & legal developments
- Scientific and technical writings for product information and related preemptions
- Regional government and statistical databases for macro analysis
- Authentic new articles, webcasts, and other related releases for market evaluation
- Internal and external proprietary databases, key market indicators, and relevant press releases for market estimates and forecasts

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | April 2025 |
| Forecast Period | 2024 - 2033 |
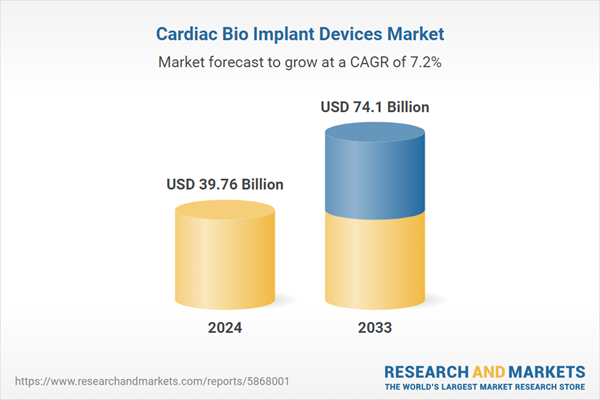
| Estimated Market Value ( USD | $ 39.76 Billion |
| Forecasted Market Value ( USD | $ 74.1 Billion |
| Compound Annual Growth Rate | 7.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 8 |