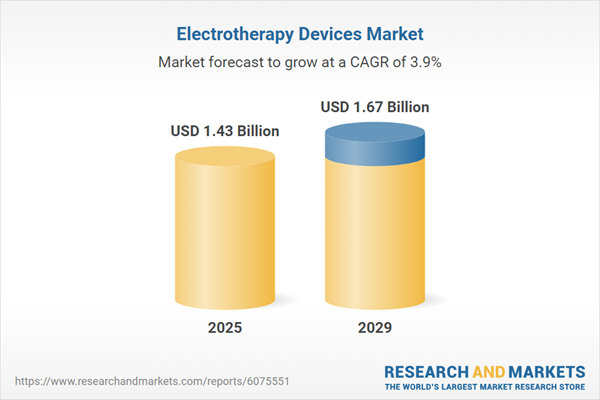
The electrotherapy devices market size has grown steadily in recent years. It will grow from $1.38 billion in 2024 to $1.43 billion in 2025 at a compound annual growth rate (CAGR) of 4.2%. The growth observed during the historic period can be attributed to increased awareness and acceptance of electrotherapy, the expansion of physical therapy and rehabilitation services, stronger clinical evidence supporting the effectiveness of electrotherapy, and the availability of favorable reimbursement policies.
The electrotherapy devices market size is expected to see steady growth in the next few years. It will grow to $1.67 billion in 2029 at a compound annual growth rate (CAGR) of 3.9%. The anticipated growth during the forecast period can be attributed to government initiatives, positive clinical outcomes demonstrating the efficacy of electrotherapy devices, the expansion of healthcare infrastructure, and an increasing demand for non-addictive pain relief alternatives. Key trends during this period include advancements in transcutaneous electrical nerve stimulation (TENS) technology, the integration of artificial intelligence and smart technologies, the development of combination therapies, and the introduction of subscription-based models.
The rising incidence of sports injuries is anticipated to drive the growth of the electrotherapy devices market in the future. Sports injuries are physical injuries that occur during athletic activities or exercise, impacting muscles, bones, joints, or other tissues. The growing number of sports injuries can be linked to factors such as early sports specialization, increased participation rates, intense training and competition, and inadequate conditioning and warm-up routines. Electrotherapy devices play a significant role in managing and treating sports injuries by aiding in pain reduction, promoting healing, and accelerating recovery. For example, in May 2023, a report by the National Safety Council, a U.S.-based non-profit organization, revealed an 8% increase in sports injuries, rising from 445,642 in 2022 to 482,886 in 2023, with the highest injury rates observed among individuals aged 15 to 24 and more injuries reported in males (276,377) compared to females (206,381). Consequently, the rise in sports injuries is fueling the expansion of the electrotherapy devices market.
Leading companies in the electrotherapy devices market are emphasizing the development of innovative solutions, such as wearable electrotherapy devices, to enhance patient outcomes by providing greater comfort. Wearable electrotherapy devices are portable devices designed to deliver electrical stimulation to the body for therapeutic purposes, such as pain relief, muscle rehabilitation, or recovery, and are worn directly on the body during use. For example, in March 2023, Zynex, a U.S.-based company specializing in electrotherapy devices, introduced the NexWave Electrotherapy Device. This innovative pain management solution offers a non-invasive alternative to traditional pain medications. The device combines transcutaneous electrical nerve stimulation (TENS) and interferential current (IFC) therapies, providing pain relief reportedly stronger than conventional TENS devices, without the side effects associated with medication. Designed for at-home use, the NexWave makes it convenient for patients recovering from surgeries or managing chronic pain.
In March 2022, Electromedical Products International (EPI), a U.S.-based medical equipment manufacturer, acquired Pulvinar Neuro for an undisclosed amount. This acquisition aims to expedite the development and commercialization of advanced neuromodulation technologies for mental health treatments. By integrating Pulvinar Neuro's research expertise with EPI's established market presence, the collaboration seeks to deliver non-invasive solutions for psychiatric conditions. Pulvinar Neuro, a U.S.-based neurotechnology company, specializes in electrotherapy devices with a focus on non-invasive brain stimulation technologies.
Major players in the electrotherapy devices market are Medtronic plc, Boston Scientific Corporation, EME Srl, Aurora Healthcare Inc., Omron Healthcare Inc., RS Medical Inc., DJO Global Inc., BTL Industries Inc., Orthofix Medical Inc., Zynex Inc., St. Jude Medical Inc., Stimwave LLC, Bioness Inc., Chattanooga Group Inc., HoMedics LLC, NeuroMetrix Inc., Mettler Electronics Corp., STYMCO Technologies Inc., Alkalife LLC, and Revitive.
North America was the largest region in the electrotherapy devices market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in electrotherapy devices report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the electrotherapy devices market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Electrotherapy devices are medical instruments designed to deliver electrical impulses to the body for therapeutic applications. They are commonly used to manage pain, stimulate muscles and nerves, enhance healing, and improve circulation. These devices utilize various methods, such as transcutaneous electrical nerve stimulation (TENS) or neuromuscular electrical stimulation (NMES), to address specific medical conditions.
The primary types of electrotherapy devices include transcutaneous electrical nerve stimulation and therapeutic ultrasound. TENS is a pain management technique that uses low-voltage electrical currents to alleviate pain by stimulating sensory nerves through electrodes placed on the skin. These devices are used for conditions such as nervous system disorders, muscle injuries, inflammation, bone growth stimulation, and general pain relief. They serve a wide range of end users, including hospitals, clinics, and rehabilitation centers.
The electrotherapy devices market research report is one of a series of new reports that provides electrotherapy devices market statistics, including electrotherapy devices industry global market size, regional shares, competitors with an electrotherapy devices market share, detailed electrotherapy devices market segments, market trends and opportunities, and any further data you may need to thrive in the electrotherapy devices industry. This electrotherapy devices market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The electrotherapy devices market consists of sales of transcutaneous electrical nerve stimulation (TENS) devices, electrical muscle stimulation (EMS) devices, and interferential current (IFC) therapy devices. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Electrotherapy Devices Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on electrotherapy devices market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for electrotherapy devices? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The electrotherapy devices market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) by Type: Transcutaneous Electrical Nerve Stimulation; Therapeutic Ultrasound2) by Application: Nervous Disease; Muscle Injury; Inflammation; Bone Growth; Pain Relief
3) by End User: Hospitals and Clinics; Rehabilitation Centers
Subsegments:
1) by Transcutaneous Electrical Nerve Stimulation (TENS): Conventional TENS Devices; Acupuncture-Like TENS Devices; Burst Mode TENS Devices2) by Therapeutic Ultrasound: Low-Intensity Therapeutic Ultrasound (LITUS) Devices; High-Intensity Focused Ultrasound (HIFU) Devices; Phonophoresis Devices
Key Companies Profiled: Medtronic plc; Boston Scientific Corporation; EME Srl; Aurora Healthcare Inc.; Omron Healthcare Inc.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Electrotherapy Devices market report include:- Medtronic plc
- Boston Scientific Corporation
- EME Srl
- Aurora Healthcare Inc.
- Omron Healthcare Inc.
- RS Medical Inc.
- DJO Global Inc.
- BTL Industries Inc.
- Orthofix Medical Inc.
- Zynex Inc.
- St. Jude Medical Inc.
- Stimwave LLC
- Bioness Inc.
- Chattanooga Group Inc.
- HoMedics LLC
- NeuroMetrix Inc.
- Mettler Electronics Corp.
- STYMCO Technologies Inc.
- Alkalife LLC
- Revitive
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 175 |
| Published | April 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 1.43 Billion |
| Forecasted Market Value ( USD | $ 1.67 Billion |
| Compound Annual Growth Rate | 3.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |