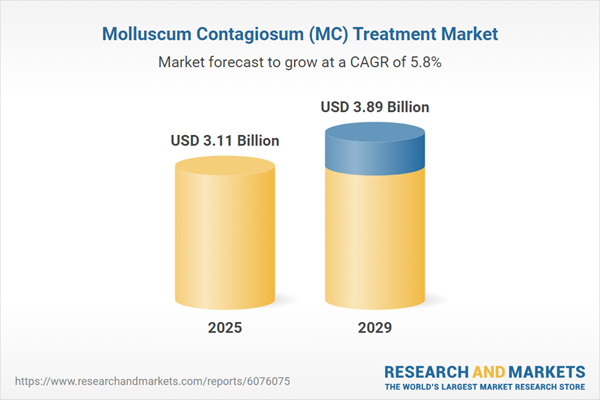
The molluscum contagiosum (MC) treatment market size is expected to see strong growth in the next few years. It will grow to $3.89 billion in 2029 at a compound annual growth rate (CAGR) of 5.8%. The growth during the forecast period can be attributed to a rising preference for non-invasive treatments, an increasing prevalence of skin diseases, growing healthcare demand, a higher incidence of chronic diseases, and the expanding availability of advanced treatment options. Key trends in the forecast period include a shift toward cosmetic treatments, the development of advanced topical delivery systems, the growing adoption of at-home treatments, advancements in cryotherapy, and a greater focus on pediatric treatment options.
The rising demand for minimally invasive treatments is driving the growth of the molluscum contagiosum treatment market. Minimally invasive treatments involve procedures with small or no incisions, offering benefits such as reduced recovery time, lower surgical risks, and improved patient outcomes compared to traditional treatments. In molluscum contagiosum treatment, methods such as cryotherapy and laser therapy effectively remove lesions while minimizing scarring and allowing for faster recovery. These treatments require fewer clinic visits and are well-suited for addressing widespread lesions. For instance, according to the American Academy of Facial Plastic and Reconstructive Surgery, minimally invasive procedures accounted for 83% of all medical treatments in 2023, totaling 35 million procedures, while traditional surgeries made up only 17%. This trend is contributing to the expansion of the molluscum contagiosum treatment market.
Leading companies in the market are focusing on innovative products, such as topical gels, to enhance treatment efficacy, improve patient compliance, and provide non-invasive solutions. Topical gels are semi-solid substances applied to the skin for localized treatment. For instance, in April 2024, Ligand Pharmaceuticals Incorporated, a US-based biopharmaceutical company, launched Pelthos Therapeutics to commercialize Zelsuvmi (brazier) topical gel, 10.3%. This is the first FDA-approved at-home treatment for molluscum contagiosum in adults and children over one year old. Zelsuvmi is a nitric oxide-releasing gel that disrupts viral replication, leading to lesion clearance. It provides a painless, non-invasive alternative to traditional in-office procedures such as cryotherapy or curettage, making it a practical and convenient option for patients.
In September 2023, Ligand Pharmaceuticals acquired Novan Inc. for $12.2 million. This acquisition aimed to preserve and enhance Novan’s commercial business and its Berdazimer gel development assets. Novan Inc. is a US-based biotechnology company specializing in treating molluscum contagiosum.
Major players in the molluscum contagiosum (mc) treatment market are Verrica Pharmaceuticals, Novan Inc., Veloce BioPharma LLC, Gurina Foundation, Competition Deep Dive, Azafaros, Polaryx Therapeutics, Abbott Medtronic, Merck & Co., Inc., Pfizer Inc., Siemens Healthcare GmbH, Novartis, Ligand Pharmaceuticals Incorporated, Aclaris Therapeutics Inc., Verrica Pharmaceuticals Inc., Nielsen BioSciences Inc., MedCara Pharmaceuticals LLC, Gurina Foundation, Veloce BioPharma LLC, Labo ACM.
North America was the largest region in the molluscum contagiosum (MC) treatment market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in molluscum contagiosum (MC) treatment report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa.
The countries covered in the molluscum contagiosum (MC) treatment market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Molluscum contagiosum (MC) treatment encompasses medical interventions aimed at managing and resolving molluscum contagiosum, a viral skin infection caused by a poxvirus. The primary goals of treatment are to eliminate characteristic lesions, prevent viral spread, and relieve associated discomfort, although the infection often resolves naturally in healthy individuals.
The primary types of molluscum contagiosum treatment target different virus subtypes, including molluscum contagiosum virus-1, virus-2, virus-3, and virus-4. Molluscum contagiosum virus-1 is the most common subtype, responsible for 75-96% of cases, primarily affecting children through direct skin contact and causing self-limiting lesions on the face, trunk, and limbs. Treatment options include topical therapy, immune modulators, and procedural treatments. Drugs are administered via various routes, such as topical and oral, and are utilized by different end users, including hospitals, dermatology clinics, homecare settings, and other healthcare facilities.
The molluscum contagiosum (MC) treatment market research report is one of a series of new reports that provides molluscum contagiosum (MC) treatment market statistics, including the molluscum contagiosum (MC) treatment industry global market size, regional shares, competitors with the molluscum contagiosum (MC) treatment market share, detailed molluscum contagiosum (MC) treatment market segments, market trends, and opportunities, and any further data you may need to thrive in the molluscum contagiosum (MC) treatment industry. This molluscum contagiosum (MC) treatment market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
The molluscum contagiosum (MC) treatment market includes revenues earned by entities by providing services such as cryotherapy, curettage, laser therapy, and photodynamic therapy, telemedicine and online consultations. The market value includes the value of related goods sold by the service provider or included within the service offering. Only goods and services traded between entities or sold to end consumers are included.
The market value is defined as the revenues that enterprises gain from the sale of goods and services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Molluscum Contagiosum (MC) Treatment Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on molluscum contagiosum (mc) treatment market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for molluscum contagiosum (mc) treatment ? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The molluscum contagiosum (mc) treatment market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Type: Molluscum Contagiosum Virus (MCV) -1; Molluscum Contagiosum Virus (MCV) -2; Molluscum Contagiosum Virus (MCV) -3; Molluscum Contagiosum Virus (MCV) -42) By Treatment Type: Topical Therapy; Immune-Modulators And Procedural Treatments
3) By Route Of Administration: Topical; Oral
4) By End User: Hospitals; Dermatology Clinics; Homecare Settings; Other End Users
Key Companies Profiled: Verrica Pharmaceuticals; Novan Inc.; Veloce BioPharma LLC; Gurina Foundation; Competition Deep Dive
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Molluscum Contagiosum (MC) Treatment market report include:- Verrica Pharmaceuticals
- Novan Inc.
- Veloce BioPharma LLC
- Gurina Foundation
- Competition Deep Dive
- Azafaros
- Polaryx Therapeutics
- Abbott Medtronic
- Merck & Co., Inc.
- Pfizer Inc.
- Siemens Healthcare GmbH
- Novartis
- Ligand Pharmaceuticals Incorporated
- Aclaris Therapeutics Inc.
- Verrica Pharmaceuticals Inc.
- Nielsen BioSciences Inc.
- MedCara Pharmaceuticals LLC
- Gurina Foundation
- Veloce BioPharma LLC
- Labo ACM
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 175 |
| Published | January 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 3.11 Billion |
| Forecasted Market Value ( USD | $ 3.89 Billion |
| Compound Annual Growth Rate | 5.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |