Speak directly to the analyst to clarify any post sales queries you may have.
Pioneering Next-Generation Cell Banking Solutions through Cutting-Edge Technologies Quality Management and Strategic Partnerships for Accelerated Bioprocessing
The landscape of biopharmaceutical manufacturing has witnessed an unprecedented emphasis on the critical role of cell banking services in ensuring consistent, safe, and high-quality clinical and commercial biologics production. As regulatory authorities intensify scrutiny on process validation and traceability, organizations are compelled to implement robust cell banking frameworks under stringent Good Manufacturing Practice conditions. These frameworks not only safeguard the integrity and genetic stability of production cell lines but also serve as the foundational bedrock for scalable manufacturing across multiple therapeutic modalities.Moreover, the integration of innovative technologies-ranging from automated cryopreservation systems to advanced bioreactor controls-has expedited the establishment of primary and working cell banks that meet evolving global standards. With each phase of product development requiring tailored cell sources, the demand for specialized cell banking services has expanded beyond traditional mammalian systems to include insect and microbial platforms. Consequently, companies are pursuing strategic alliances to leverage expertise in process optimization, regulatory compliance, and technology transfer, thus reducing time-to-clinic and enhancing overall competitiveness.
In this executive summary, we distill the core developments shaping the cell banking domain, highlighting transformative shifts, regulatory influences, and strategic segmentation insights. Our goal is to equip decision-makers with a comprehensive understanding of operational best practices, regional nuances, and actionable recommendations poised to drive sustainable growth in the rapidly evolving biomanufacturing ecosystem.
Unprecedented Technological and Regulatory Transformations Reshaping the Cell Banking Landscape and Unlocking New Opportunities in Biotherapeutic Production
Over the past decade, the cell banking sector has been reshaped by a confluence of technological breakthroughs and regulatory recalibrations that are redefining operational excellence. Automation technologies now permit end-to-end control of cryopreservation workflows, dramatically reducing manual intervention and enhancing reproducibility. In parallel, the adoption of single-use bioreactor systems has lowered cross-contamination risks and enabled more flexible scale-up pathways across diverse cell types.Furthermore, the integration of artificial intelligence and machine learning algorithms has begun to transform cell line characterization, enabling predictive assessments of viability, productivity, and genetic drift. This predictive capability facilitates proactive quality interventions and streamlines regulatory submissions by providing robust, data-driven evidence of process consistency. Consequently, organizations can balance stringent compliance with accelerated development timelines, bridging the gap between discovery and commercial launch.
Regulatory bodies have also introduced new guidance documents that emphasize risk-based approaches to cell line establishment and storage. These guidelines reinforce the need for comprehensive documentation, contamination control, and traceable chain-of-custody protocols. As a result, service providers are investing in high-throughput analytical platforms and modular facility designs to meet global standards with greater agility. Combined, these advances underscore a transformative shift toward a more resilient and efficient cell banking paradigm, where technology and regulation converge to unlock novel biotherapeutic opportunities.
Assessing the Far-Reaching Consequences of 2025 US Tariff Adjustments on Cell Banking Operations and Global Supply Chain Dynamics Strategic Implications
The announcement of revised US tariff measures slated for implementation in 2025 has introduced a complex layer of strategic decision-making for cell banking service providers and end users alike. As critical raw materials, consumables, and specialized equipment face elevated import duties, supply chain managers are re-evaluating vendor portfolios and exploring localized sourcing alternatives. This shift toward regional procurement is already influencing inventory planning, lead-time estimations, and budget allocations across R&D and commercial manufacturing operations.In addition, the cumulative financial burden imposed by these tariffs is prompting organizations to examine their cost structures, particularly in relation to sterile processing equipment and single-use consumable components sourced from affected trade regions. Consequently, providers offering in-house production of key reagents and equipment assemblies are gaining traction as attractive partners. This re-orientation toward vertically integrated supply chains can mitigate exposure to external pricing shocks and enhance overall resilience.
Moreover, the ripple effects of these tariff adjustments extend beyond cost considerations to include strategic realignments in cross-border collaborations. Research alliances that once leveraged cost arbitrage may now pivot toward joint ventures or technology licensing agreements to preserve scalability while containing expenditure. Ultimately, the 2025 tariff framework underscores the imperative for dynamic supply chain strategies that balance cost optimization with uninterrupted access to critical cell banking resources.
Comprehensive Breakdown of Cell Banking Market Segmentation Drivers Spanning Cell Type Product Variants Application End Users and Operational Modalities
A nuanced understanding of cell banking market segmentation is vital for tailoring service offerings to diverse application needs. When examining the landscape through the prism of cell type, insect platforms such as Sf21, Sf9, and Tn5 are indispensable for certain vaccine and viral vector workflows, complementing mammalian systems based on CHO, HEK293, hybridoma, and NS0 lines. Microbial hosts, encompassing Bacillus or E coli for bacterial expression and Pichia pastoris or Saccharomyces for yeast-derived products, add further versatility in enzyme and small protein manufacturing. Appreciating these biological distinctions is foundational to matching cell bank solutions with specific development objectives.Transitioning to the realm of product offerings, services bifurcate into primary cell bank creation-establishing a master reference repository-and working cell bank generation, which ensures sufficient downstream material for process validation and scale-up. The selection of either bank type influences storage, quality control, and regulatory submission strategies, with each requiring distinct validation packages and release testing protocols. Meanwhile, the dichotomy between commercial production and R&D applications drives differential demand for custom formulation, stability studies, and back-up storage solutions.
End users further diversify the marketplace, encompassing academic institutes and research laboratories, where Government Research Institutes and universities pursue foundational research, as well as biopharmaceutical companies that emphasize GMP compliance for clinical pipelines. Contract research organizations play a pivotal role by offering outsourced banking capabilities to streamline client portfolios. Additionally, the choice between allogeneic and autologous cell sources, including sibling and unrelated donor options for the former, underscores patient-specific versus broad-spectrum therapeutic strategies.
Moreover, the intended scale of use dictates operational frameworks: early-stage clinical use encompassing Phase I and combined Phase II/III trials requires flexible, small-batch production, whereas commercial use demands large-scale, standardized bank management. Selection of storage technologies, from cryopreservation tanks to lyophilization modalities, interacts with these scale requirements to optimize stability and logistics. Process type considerations draw a line between automated systems offering high throughput and manual workflows suited to bespoke protocols. Finally, distribution channels range from direct sales to third-party distributors, each presenting unique implications for quality agreements, cold-chain logistics, and after-sales support.
Strategic Examination of Regional Dynamics and Growth Opportunities in Cell Banking across Americas Europe Middle East Africa and Asia Pacific Markets
Regional dynamics in cell banking reflect distinct market drivers and operational imperatives across the Americas, Europe, Middle East & Africa, and Asia-Pacific. In the Americas, established biomanufacturing hubs benefit from advanced infrastructure, favorable regulatory harmonization, and a strong ecosystem of contract service providers. These factors contribute to rapid adoption of next-generation technologies and foster competitive innovation clusters focused on gene therapy and personalized medicine applications.Conversely, Europe, the Middle East & Africa region is characterized by stringent regulatory frameworks and robust quality oversight. This environment encourages service providers to invest in modular cleanroom facilities and digital batch tracking systems to maintain compliance across multiple jurisdictions. Moreover, collaboration between research consortia and industry suppliers is increasingly common, enabling streamlined tech transfers and shared access to high-throughput analytical platforms.
Meanwhile, Asia-Pacific is emerging as a dynamic growth frontier, underpinned by lower manufacturing costs, government incentives for biotech scaling, and expanding academic-industry partnerships. Local service providers are enhancing their capabilities through strategic technology transfers, capacity expansions, and workforce training initiatives. Although market fragmentation presents hurdles, the rapidly evolving regulatory landscapes in key markets are progressively aligning with global GMP standards, opening avenues for cross-border collaboration and export opportunities.
Competitive Dynamics and Innovation Pathways Forged by Leading Cell Banking Service Providers Leveraging Strategic Alliances and Advanced Technologies
Leading cell banking service providers are differentiating themselves through a combination of strategic alliances, technological investments, and tailored service portfolios. Some organizations have forged partnerships with single-use system manufacturers to co-develop integrated cryopreservation-to-thaw workflows that reduce contamination risk and accelerate time-to-delivery. Others have prioritized in-house analytical capabilities, deploying high-resolution genomic and proteomic platforms to validate cell line integrity and streamline regulatory dossier preparation.Further, a number of prominent firms are extending their geographic footprint by establishing regional centers of excellence, thereby offering localized support and minimizing cross-border shipping challenges. This approach not only enhances customer service but also aligns with emerging tariff considerations by reducing import dependencies. Meanwhile, those with robust digital infrastructures are implementing cloud-based traceability solutions, enabling real-time monitoring of storage conditions, audit trails, and batch release notifications.
While some players concentrate on proprietary cell line development and licensing models to secure recurring revenue streams, others adopt flexible contract frameworks that cater to both early-stage researchers and large-scale manufacturers. Through targeted acquisitions and joint ventures, these companies are expanding their end-to-end capabilities, ensuring clients can transition seamlessly from cell line generation to commercial release under consistent GMP oversight.
Pragmatic Action Plans for Industry Leadership to Harness Emerging Trends Optimize Operations and Drive Sustainable Growth in Cell Banking Services
Industry leaders seeking to capitalize on the evolving cell banking environment should prioritize investments in automation to enhance throughput and reduce variability. By integrating robotic handling and in-line viability assessment tools, organizations can minimize manual errors and accelerate bank qualification cycles. In addition, diversifying supplier bases for critical reagents and single-use components will bolster supply chain resilience amidst shifting tariff policies.Furthermore, cultivating strategic collaborations with technology developers and academic research centers can unlock early access to innovative analytical methodologies, positioning service providers at the forefront of regulatory compliance trends. Embracing digital traceability platforms will not only improve audit readiness but also facilitate real-time decision support, enabling rapid responses to process deviations.
Moreover, adopting a modular facility design philosophy affords scalable expansion opportunities without substantial capital expenditures. Leaders should also consider developing hybrid pricing models that balance fixed-fee services for routine applications with value-based pricing for complex, high-stakes projects. Altogether, these actionable steps will help organizations strengthen their competitive posture, optimize operational efficiency, and achieve sustainable growth in a rapidly maturing market.
Rigorous Multi-Stage Research Methodology Integrating Primary Interviews Secondary Intelligence and Technical Validation for Robust Cell Banking Market Insights
The research underpinning these insights employed a rigorous multi-stage approach, beginning with in-depth interviews of key opinion leaders across biopharmaceutical companies, contract research organizations, and academic research institutions. These primary conversations were complemented by site visits to state-of-the-art cell banking facilities, enabling direct observation of automated workflows and emerging storage technologies.Subsequently, a thorough review of regional regulatory frameworks was conducted to map compliance imperatives across North America, EMEA, and Asia-Pacific jurisdictions. Secondary sources, including peer-reviewed publications, patent filings, and industry conference proceedings, were triangulated to validate technological trends and adoption patterns. Analytical models assessed supply chain dependencies and tariff impact scenarios without attempting quantitative forecasting, focusing instead on thematic implications.
Finally, a technical validation roundtable convened subject matter experts in cell line development, process engineering, and quality assurance to refine our findings and ensure actionable relevance. This multi-faceted methodology provides a balanced perspective on the strategic, operational, and regulatory facets shaping the cell banking services landscape.
Conclusive Reflections on Evolving Cell Banking Strategies Technologies and Market Forces Guiding the Biopharmaceutical Ecosystem Forward
The culmination of this analysis underscores a pivotal moment for cell banking service providers as they navigate a landscape defined by technological innovation, regulatory evolution, and shifting global trade dynamics. Strategic investments in automation and digital traceability, coupled with agile supply chain strategies, will be essential to mitigate cost pressures and maintain uninterrupted service delivery.Moreover, a clear segmentation approach-tailoring solutions to distinct cell types, end users, and application scales-enables precise alignment with client needs and fosters long-term partnerships. Regional variations in regulatory requirements and market maturity present both challenges and opportunities; recognizing these nuances is critical for expanding geographic reach and optimizing localized operations.
By adopting collaborative innovation models and embracing modular facility expansions, organizations can balance scalability with cost efficiency. Ultimately, success in this domain will hinge on the ability to integrate advanced technologies, uphold rigorous quality standards, and deliver customized, end-to-end cell banking solutions that accelerate biopharmaceutical development.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Service Type
- Cell Banking & Storage
- Cell Line Development & Characterization
- Regulatory Support & Documentation
- Testing & Quality Control
- Adventitious Agent Testing
- Genetic Stability Testing
- Identity Testing
- Purity Testing
- Sterility Testing
- Cell Type
- Insect Cells
- Mammalian Cells
- CHO Cells
- HEK293 Cells
- NS0/Sp2 Cells
- Vero Cells
- Microbial Cells
- Bacterial Cells
- Yeast Cells
- Stem Cells
- Embryonic Stem Cells
- Induced Pluripotent Stem Cells (iPSCs)
- Mesenchymal Stem Cells (MSCs)
- Cell Bank Type
- End-of-Production Cell Bank (EOPCB)
- Master Cell Bank (MCB)
- Research Cell Bank (RCB)
- Working Cell Bank (WCB)
- Application
- Biopharmaceutical Production
- Gene & Cell Therapies
- Monoclonal Antibodies
- Recombinant Proteins
- Vaccines
- Cell Therapy
- Drug Discovery & Development
- Tissue Engineering & Regenerative Medicine
- Biopharmaceutical Production
- End User
- Academic & Research Institutes
- Contract Research & Manufacturing Organizations
- Hospitals & Clinics
- Pharmaceutical & Biotechnology Companies
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- 53Biologics
- Austrianova
- Cell Culture Company, LLC
- Charles River Laboratories
- Clean Cells
- Eurofins Scientific Limited
- ExcellGene SA
- Goodwin Biotechnology Inc.
- Kaneka Eurogentec S.A.
- Lonza Group Ltd.
- Merck KGaA
- NAOBIOS
- PackGene Biotech lnc.
- ProBio Biotech Corporation
- RoslinCT
- SGS Group
- SK pharmteco Inc.
- Takara Bio Inc.
- Texcell SA
- uBriGene Biosciences International Co.
- ViruSure GmbH
- Wacker Chemie AG
- WuXi Biologics
- OmniaBio
- eXmoor pharma concepts ltd
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this GMP Cell Banking Services Market report include:- 53Biologics
- Austrianova
- Cell Culture Company, LLC
- Charles River Laboratories
- Clean Cells
- Eurofins Scientific Limited
- ExcellGene SA
- Goodwin Biotechnology Inc.
- Kaneka Eurogentec S.A.
- Lonza Group Ltd.
- Merck KGaA
- NAOBIOS
- PackGene Biotech lnc.
- ProBio Biotech Corporation
- RoslinCT
- SGS Group
- SK pharmteco Inc.
- Takara Bio Inc.
- Texcell SA
- uBriGene Biosciences International Co.
- ViruSure GmbH
- Wacker Chemie AG
- WuXi Biologics
- OmniaBio
- eXmoor pharma concepts ltd
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 190 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
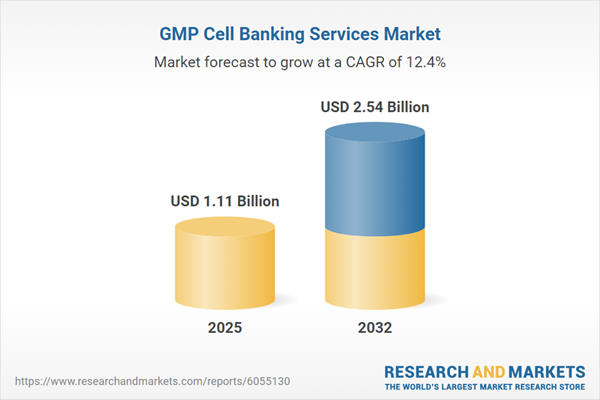
| Estimated Market Value ( USD | $ 1.11 Billion |
| Forecasted Market Value ( USD | $ 2.54 Billion |
| Compound Annual Growth Rate | 12.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |