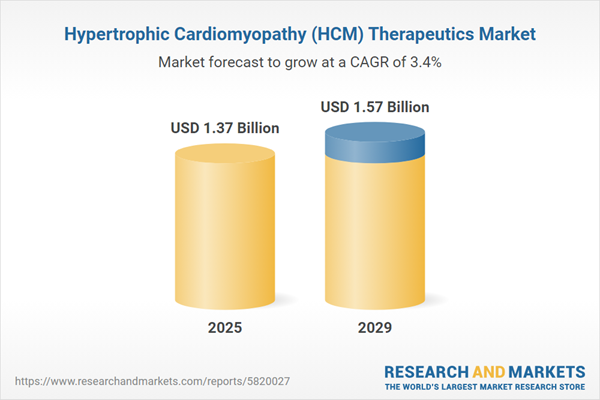
The hypertrophic cardiomyopathy (HCM) therapeutics market size is expected to see steady growth in the next few years. It will grow to $1.57 billion in 2029 at a compound annual growth rate (CAGR) of 3.4%. The growth in the forecast period can be attributed to genetic research and personalized medicine, emerging therapeutic modalities, collaborative research initiatives, patient-centric healthcare models, local health initiatives. Major trends in the forecast period include advancements in targeted therapies, personalized medicine and genetic testing, drug pipeline expansion, collaborations and partnerships, patient-centric approaches.
The growth of the hypertrophic cardiomyopathy (HCM) therapeutics market is anticipated to be propelled by the increasing prevalence of cardiovascular diseases (CVD). CVD refers to conditions affecting the heart or blood vessels, encompassing irregularities such as atrial fibrillation, ventricular fibrillation, and atrioventricular block, necessitating prolonged monitoring. Therapeutics for hypertrophic cardiomyopathy primarily aim to alleviate symptoms and prevent sudden cardiac death in high-risk patients. According to the October 2022 report from the Center for Disease Control and Prevention (CDCP), one person succumbs to cardiovascular disease in the United States every 36 seconds, contributing to nearly 836,546 deaths in the country alone in 2021. Thus, the rising incidence of CVD serves as a driving force for the hypertrophic cardiomyopathy (HCM) therapeutics market.
The surge in clinical trials is expected to further drive the growth of the hypertrophic cardiomyopathy (HCM) therapeutics market. Clinical trials, systematic investigations assessing the safety, efficacy, and potential side effects of new medical treatments, drugs, or interventions, play a crucial role in evaluating the effectiveness of Hypertrophic Cardiomyopathy (HCM) Therapeutics. As of May 2023, there were 452,604 registered clinical trials globally on ClinicalTrials.gov, as reported by Xtalks, indicating a notable increase from the 365,000 trials recorded in early 2021. Therefore, the escalation in clinical trials is a significant factor propelling the hypertrophic cardiomyopathy (HCM) therapeutics market.
A key trend gaining traction in the hypertrophic cardiomyopathy (HCM) therapeutics market is product innovation. Major players in the cardiomyopathy therapeutics sector are actively involved in developing innovative products to enhance their market position. In April 2022, Bristol Myers Squibb, a U.S.-based biopharmaceutical company, introduced Camzyos (mavacamten), an oral small-molecule cardiac myosin inhibitor for treating hypertrophic cardiomyopathy (HCM) and heart diastolic dysfunction diseases. FDA-approved for adults with symptomatic class II-III obstructive hypertrophic cardiomyopathy, Camzyos is the first and only allosteric and reversible inhibitor of cardiac myosin, addressing the underlying pathophysiology of obstructive HCM by controlling the number of myosin heads entering power-generating states on actin.
Key players in the hypertrophic cardiomyopathy (HCM) therapeutics market are concentrating on innovations, such as novel gene-targeting therapies, to offer patients advanced treatment options that enhance heart function and alleviate the symptoms of both obstructive and non-obstructive HCM. Mavacamten (Camzyos) is a drug specifically designed to treat symptomatic obstructive hypertrophic cardiomyopathy (HOCM) by reducing excessive contractions of the heart muscle, thereby improving heart function and easing symptoms. For example, in May 2022, Bristol Myers Squibb (BMS), a biopharmaceutical company based in the U.S., launched mavacamten (Camzyos), which is available in capsule strengths of 2.5 mg, 5 mg, 10 mg, and 15 mg for the treatment of adults with symptomatic New York Heart Association (NYHA) class 2-3 obstructive hypertrophic cardiomyopathy (HOCM) to enhance functional capacity and reduce symptoms.
In November 2023, Merck Sharp & Dohme, a U.S.-based pharmaceutical company, acquired Caraway Therapeutics, Inc. for an undisclosed amount. This acquisition enables Merck to utilize its industry-leading research and development capabilities to advance innovative therapies for genetically defined neurodegenerative and rare diseases, with the goal of developing disease-modifying treatments that can significantly impact disease progression. Caraway Therapeutics is a U.S.-based biopharmaceutical firm focused on developing small-molecule therapeutics for rare and neurodegenerative diseases.
Major companies operating in the hypertrophic cardiomyopathy (HCM) therapeutics market include Merck & Co. Inc., Pfizer Inc., Sanofi SA, Teva Pharmaceutical Industries Ltd., Novartis AG, AstraZeneca plc, Gilead Sciences Inc., Bayer AG, Correvio Pharma Corp., Advanz Pharma Corp., Lupin Limited, Boston Scientific Corporation, Bristol-Myers Squibb Company, Johnson & Johnson, Medtronic plc, Cardiome Pharma Corp., Cytokinetics Inc., Daiichi Sankyo Company Limited, GlaxoSmithKline plc., HUYA Bioscience International LLC, Ionis Pharmaceuticals Inc., Kowa Company Ltd., Luitpold Pharmaceuticals Inc., Menarini Group, Mitsubishi Tanabe Pharma Corporation, Nippon Shinyaku Co. Ltd., Otsuka Holdings Co. Ltd., Servier Laboratories.
North America was the largest region in the hypertrophic cardiomyopathy (HCM) therapeutics market in 2024. Asia-Pacific is expected to be the fastest-growing region in the hypertrophic cardiomyopathy (HCM) therapeutics market report during the forecast period. The regions covered in the hypertrophic cardiomyopathy (hcm) therapeutics market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the hypertrophic cardiomyopathy (hcm) therapeutics market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Hypertrophic cardiomyopathy (HCM) therapeutics refer to the treatment of a condition characterized by the abnormal thickening (hypertrophy) of the heart muscle, leading to impaired blood-pumping efficiency. The goal of hypertrophic cardiomyopathy therapeutics is to alleviate symptoms and prevent sudden cardiac death, particularly in high-risk patients.
The primary drug types used in hypertrophic cardiomyopathy therapeutics include antiarrhythmic agents, anticoagulants, beta-adrenergic blocking agents, calcium channel blockers, and others. Antiarrhythmic agents are medications designed to address abnormal heart rhythms or arrhythmias. Additionally, various devices, such as defibrillators, pacemakers, and others, are employed in hospitals and clinics as part of the therapeutic approach to managing hypertrophic cardiomyopathy.
The hypertrophic cardiomyopathy (HCM) therapeutics market research report is one of a series of new reports that provides hypertrophic cardiomyopathy (HCM) therapeutics market statistics, including hypertrophic cardiomyopathy (HCM) therapeutics industry global market size, regional shares, competitors with hypertrophic cardiomyopathy (HCM) therapeutics market share, detailed hypertrophic cardiomyopathy (HCM) therapeutics market segments, market trends, and opportunities, and any further data you may need to thrive in the hypertrophic cardiomyopathy (HCM) therapeutics industry. This hypertrophic cardiomyopathy (HCM) therapeutics market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
The hypertrophic cardiomyopathy (HCM) therapeutics market consists of revenues earned by entities by provide progressive disease management, physical therapy (PT), and occupational therapy. The market value includes the value of related goods sold by the service provider or included within the service offering. The hypertrophic cardiomyopathy (HCM) therapeutics market also consists of sales of disopyramide, wearable heart monitoring devices, and cardiac rhythm management (CRM) devices which are used in providing hypertrophic cardiomyopathy (HCM) therapeutics services. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Hypertrophic Cardiomyopathy (HCM) Therapeutics Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on hypertrophic cardiomyopathy (hcm) therapeutics market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for hypertrophic cardiomyopathy (hcm) therapeutics ? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The hypertrophic cardiomyopathy (hcm) therapeutics market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- the market characteristics section of the report defines and explains the market.
- the market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- the forecasts are made after considering the major factors currently impacting the market. These include:
- the forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- the regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- the competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- the trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) by Drug Type: Antiarrhythmic Agents; Anticoagulants; Beta Adrenergic Blocking Agents; Calcium Channel Blockers; Other Drug Types2) by Device Type: Defibrillators; Pacemakers; Other Device Types
3) by End User: Hospitals; Clinics
Subsegments:
1) by Antiarrhythmic Agents: Class I Agents; Class II Agents; Class III Agents; Class IV Agents2) by Anticoagulants: Vitamin K Antagonists; Direct Oral Anticoagulants (DOACs); Low Molecular Weight Heparins (LMWH)
3) by Beta Adrenergic Blocking Agents: Non-Selective Beta Blockers; Selective Beta Blockers
4) by Calcium Channel Blockers: Dihydropyridines; Non-Dihydropyridines
5) by Other Drug Types: Myosin Inhibitors; Antimetabolites; Other Novel Agents
Key Companies Mentioned: Merck & Co. Inc.; Pfizer Inc.; Sanofi SA; Teva Pharmaceutical Industries Ltd.; Novartis AG
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
Some of the major companies featured in this Hypertrophic Cardiomyopathy (HCM) Therapeutics market report include:- Merck & Co. Inc.
- Pfizer Inc.
- Sanofi SA
- Teva Pharmaceutical Industries Ltd.
- Novartis AG
- AstraZeneca plc
- Gilead Sciences Inc.
- Bayer AG
- Correvio Pharma Corp.
- Advanz Pharma Corp.
- Lupin Limited
- Boston Scientific Corporation
- Bristol-Myers Squibb Company
- Johnson & Johnson
- Medtronic plc
- Cardiome Pharma Corp.
- Cytokinetics Inc.
- Daiichi Sankyo Company Limited
- GlaxoSmithKline plc.
- HUYA Bioscience International LLC
- Ionis Pharmaceuticals Inc.
- Kowa Company Ltd.
- Luitpold Pharmaceuticals Inc.
- Menarini Group
- Mitsubishi Tanabe Pharma Corporation
- Nippon Shinyaku Co. Ltd.
- Otsuka Holdings Co. Ltd.
- Servier Laboratories
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | February 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 1.37 Billion |
| Forecasted Market Value ( USD | $ 1.57 Billion |
| Compound Annual Growth Rate | 3.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 28 |