Immune Thrombocytopenia Market Overview
Immune thrombocytopenia is an autoimmune disease characterized by the immune system destroying the body's platelets. Excessive reduction in platelet count can lead to severe bruising, bleeding gums, and internal bleeding issues may arise. The condition can occur in adults and children and may be considered acute or chronic, based on the duration of symptoms and frequency of relapses. The market is driven by growing awareness, emphasis on early diagnostics, and treatment of the condition. A rise in the geriatric population can also be considered a major factor driving the growth of the market.Immune Thrombocytopenia Market Growth Drivers
Growing Prevalence of Related Autoimmune Disorders
Immune thrombocytopenia can be associated with other autoimmune diseases such as lupus and rheumatoid arthritis. The 2023 NCBI report indicated that lupus has a prevalence rate of 72.1 to 74.4 per 100,000 individuals, with an incidence rate of 5.6 per 100,000 person-years. The rise in autoimmune disorders can contribute to significant market growth in the forecast period.Rise in Research Initiatives and Clinical Trials to Meet Rising Immune Thrombocytopenia Market Demand
In June 2024, Takeda Pharmaceuticals reported that mezagitamab, an experimental drug, showed encouraging outcomes in Phase 2b trials for immune thrombocytopenia. The experiments demonstrated that mezagitamab, when given subcutaneously in different amounts, elevated the platelet levels and the effect lasted for weeks after treatment. This study occurred at various sites and evaluated the safety and effectiveness in individuals suffering from chronic or persistent immune thrombocytopenia. Increasing research activities by leading companies is one of the notable market trends, expected to impact the market positively.Immune Thrombocytopenia Market Trends
Several trends and developments are being observed in the market to enhance the current situation. Some of the noteworthy trends are as follows:Focus on Patient-Centric Therapies to Enhance Treatment Experiences
Companies are creating treatments with personalized dosing and administration. This focus on the patient is helping a diverse set of affected individuals catering to their specific needs, and leading to better treatment adherence and outcomes in the market.Rising Geriatric Population
An increase in the aging population has resulted in a higher risk of autoimmune disorders like immune thrombocytopenia, thereby creating a high demand for autoimmune treatment alternatives. This trend is particularly noteworthy in developed countries where healthcare systems are capable of sustaining advanced therapies for the geriatric population.Innovative Therapies in the Pipeline to Boost Immune Thrombocytopenia Market Value
Pharmaceutical firms are broadening their range of treatments for immune thrombocytopenia, with many therapies and drugs undergoing clinical trials. The expanding pipeline shows a long-term dedication to meeting patient needs, offering optimism for future market expansion as new treatments address the challenges of managing immune thrombocytopenia.Rising Investments in Emerging Markets
Healthcare companies are putting more money into developing markets, notably in the Asia Pacific region, where advancements in healthcare infrastructure and increased awareness of diseases are leading to new opportunities.Immune Thrombocytopenia Market Segmentation
“Immune Thrombocytopenia Market Report and Forecast 2025-2034” offers a detailed analysis of the market based on the following segments:Market Breakup by Treatment Type
- Thrombopoietin Receptor Agonists
- Corticosteroids
- Intravenous Immunoglobulins
- Others
Market Breakup by Disease Type
- Acute
- Chronic
Market Breakup by Route of Administration
- Oral
- Injectable
Market Breakup by Distribution Channel
- Hospital Pharmacy
- Online Pharmacy
- Retail Pharmacy
Market Breakup by Region
- United States
- EU-4 and the United Kingdom
- Germany
- France
- Italy
- Spain
- United Kingdom
- Japan
- India
Immune Thrombocytopenia Market Share
Chronic Segment is Anticipated to Undergo Substantial Growth in the Share by Disease Type
Based on disease type, the market is divided into acute and chronic immune thrombocytopenia. Among these, the chronic segment is expected to dominate the market as it needs extensive treatment and management. Unlike acute immune thrombocytopenia, chronic immune thrombocytopenia demands continuous therapeutic focus. Thus, there is a focus on speeding up research, significantly impacting the immune thrombocytopenia treatment landscape.Market Segmentation Based on the Route of Administration Set to Witness Substantial Growth
The market segmentation by route of administration includes orals and injectables. Out of these, oral treatments are expected to lead the market as they are easy to use, convenient, and help patients stick to their medication plans, especially those needing long-term treatment. Oral treatments are becoming more popular for chronic immune thrombocytopenia as patients prefer an easier route of administration.Immune Thrombocytopenia Market Analysis by Region
The market is segmented by region including the United States, EU-4 (Germany, France, Italy, Spain), the United Kingdom, Japan, and India. Among these, the United States is expected to dominate the market share which can be attributed to the high incidence of the condition along with substantial funding and investment for research. The market in this region is influenced by factors such as a growing elderly population and lifestyle-related risks. Government support and a strong healthcare system boost market growth by leading clinical trials and discovering treatments like targeted therapies and immunotherapies. There is also a rising emphasis on early diagnosis and personalized treatments, which fuels growth further. Europe also holds a considerable market share, owing to the presence of numerous research and academic institutions.Leading Players in the Immune Thrombocytopenia Market
The key features of the market report include patent analysis, clinical trials analysis, grants analysis, funding and investment analysis, and strategic initiatives by the leading players. The major companies are:Pfizer Inc
Pfizer, founded in 1849 and based in New York City is one of the leading pharmaceutical companies globally. The company has a strong focus on immunology and hematology. In the immune thrombocytopenia market, Pfizer offers Tavalisse (fostamatinib), an oral spleen tyrosine kinase (SYK) inhibitor for treating chronic immune thrombocytopenia in adults. The company continues to invest in research and development to expand its immune thrombocytopenia treatment pipeline, including novel biologics and immunotherapies to improve patient outcomes.F. Hoffmann-La Roche Ltd
Founded in 1896 and headquartered in Basel, Switzerland, Roche’s portfolio includes MabThera/Rituxan (rituximab), a monoclonal antibody used for patients with refractory immune thrombocytopenia. This treatment targets CD20-positive B-cells and has proven effective for chronic immune thrombocytopenia patients.Amgen Inc
Founded in 1980 and based in Thousand Oaks, California, Amgen is known for its biologics that treat hematology and autoimmune diseases. The company offers Nplate (romiplostim), a thrombopoietin receptor agonist that helps increase platelet counts in adults with chronic immune thrombocytopenia.Novartis AG
Founded in 1996 and based in Basel, Switzerland, Novartis offers Promacta (eltrombopag), a thrombopoietin receptor agonist used for treating chronic immune thrombocytopenia in adults and children unresponsive to other treatments. Promacta is available in oral and pediatric formulations, making it suitable for different age groups and treatment needs.Other companies include Rigel Pharmaceuticals, Inc., Dova Pharmaceuticals Inc., Grifols S.A, CSL Limited, Octapharma AG, Intas Pharmaceuticals Ltd, and Saol Therapeutics.
Key Questions Answered in the Immune Thrombocytopenia Market
- What was the immune thrombocytopenia market value in 2024?
- What is the immune thrombocytopenia market forecast outlook for 2025-2034?
- What are the regional markets covered in the report?
- What is the market segmentation based on the treatment type?
- What is the market segmentation based on the disease type?
- What is the market breakup by route of administration?
- What is the market breakup based on the distribution channel?
- What major factors aid the immune thrombocytopenia market demand?
- What are the market's major drivers, opportunities, and restraints?
- Which regional market is expected to lead the market share in the forecast period?
- Which country is expected to experience expedited growth during the forecast period?
- What are the major immune thrombocytopenia market trends?
- How does the rise in the geriatric population impact the market size?
- Who are the key players in the immune thrombocytopenia market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Pfizer Inc.
- F. Hoffmann-La Roche Ltd.
- Amgen Inc.
- Novartis AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 400 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
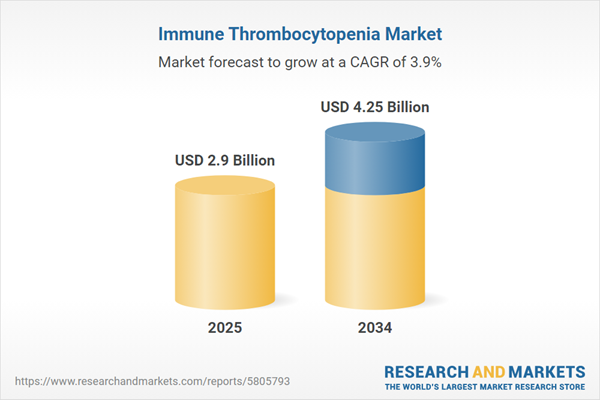
| Estimated Market Value ( USD | $ 2.9 Billion |
| Forecasted Market Value ( USD | $ 4.25 Billion |
| Compound Annual Growth Rate | 3.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 4 |