Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
With India’s growing focus on personalized medicine, RNA therapeutics are emerging as promising solutions for conditions like cancer, cardiovascular diseases, and rare genetic disorders. The market benefits from significant R&D investments by biopharma companies, a supportive regulatory environment, and cost-effective manufacturing capabilities, particularly in regions like Maharashtra and Gujarat, which serve as biotech and pharmaceutical hubs. Additionally, collaborations between Indian firms and global players are accelerating innovation and commercialization, enabling the introduction of cutting-edge RNA-based treatments.
However, the market also faces challenges such as high development costs, limited local expertise in advanced RNA technologies, and infrastructure gaps in smaller cities. Ensuring the affordability and accessibility of RNA therapeutics for the vast Indian population remains a critical hurdle. Moreover, competition from traditional therapies and the complex regulatory framework for novel biologics can delay market entry. Despite these challenges, the rising demand for precision medicine, coupled with India’s robust pharmaceutical manufacturing landscape, positions the RNA therapeutics market for significant growth in the coming years.
Key Market Drivers
Growing Prevalence of Genetic and Chronic Diseases
The increasing prevalence of genetic and chronic diseases in India is a key driver for the RNA Therapeutics Market. Genetic disorders, such as rare diseases, congenital malformations, and enzyme deficiencies, pose significant challenges to India’s healthcare system, necessitating advanced therapeutic solutions like RNA-based treatments. The substantial burden of rare and inherited diseases highlights the need for personalized medicine, particularly as traditional treatment approaches fail to address the underlying genetic causes effectively. Approximately 70 million individuals in India are affected by known rare diseases, accounting for about one-fifth of the global rare disease population. Moreover, annually, around 500,000 infants are born with congenital malformations, 400,000 with glucose-6-phosphate dehydrogenase (G6PD) deficiency, and 10,000 with amino acid disorders in India.Chronic diseases further exacerbate India’s healthcare challenges, driving demand for innovative treatments. Cardiovascular diseases (CVDs) remain a leading cause of mortality, with India facing a higher CVD burden than the global average. Similarly, the rapid rise in diabetes prevalence emphasizes the urgent need for advanced therapeutics to manage and mitigate disease progression. RNA therapeutics offer significant promise in tackling such chronic conditions by targeting disease pathways more precisely than conventional therapies. The age-standardized CVD death rate in India is 272 per 100,000 population, significantly higher than the global average of 235 per 100,000 population. Moreover, according to the ICMR INDIAB study (2023), the prevalence of diabetes in India has reached 10.1 crores, placing immense pressure on the healthcare system.
Key Market Challenges
Regulatory and Clinical Challenges
One of the significant challenges facing the RNA therapeutics market in India is the complex regulatory framework and the stringent requirements for clinical trials. Developing RNA-based therapies, such as mRNA vaccines, RNA interference (RNAi) therapeutics, and antisense oligonucleotides (ASOs), involves navigating a multi-layered approval process that can delay product development and commercialization. The lack of specific guidelines tailored to RNA therapeutics in India’s regulatory system adds an extra layer of complexity. Unlike traditional drugs, RNA-based therapies often have unique mechanisms of action and require innovative delivery systems, which may not align well with existing regulatory pathways.Moreover, conducting large-scale clinical trials in India poses challenges due to the diversity of the population, logistical constraints, and the need for specialized infrastructure. RNA therapeutics are still a relatively new field, and ensuring compliance with global standards for safety, efficacy, and manufacturing quality can be resource-intensive for companies. The cost of clinical trials, combined with the need for advanced research facilities and skilled professionals, further complicates the market landscape. Additionally, the evolving nature of RNA technology means companies must continuously adapt to changing international guidelines, which can strain smaller biotech firms or startups with limited resources.
These regulatory and clinical hurdles create bottlenecks in bringing innovative RNA therapies to market, slowing down the growth potential of the industry. Addressing these challenges will require collaboration between regulatory authorities, industry players, and academic institutions to establish clear, streamlined, and supportive frameworks for RNA-based therapeutics in India.
Key Market Trends
Advancements in Biotechnology and Genomics
The rapid advancements in biotechnology and genomics are shaping the RNA therapeutics market in India, positioning it at the forefront of modern healthcare innovation. Breakthroughs in genomic research have significantly enhanced the understanding of gene expression, disease pathways, and molecular mechanisms, paving the way for targeted RNA-based solutions. The integration of cutting-edge technologies such as next-generation sequencing (NGS), CRISPR-Cas9 gene editing, and synthetic biology has accelerated the development of RNA therapeutics, enabling researchers to identify and address genetic mutations and molecular targets more effectively.In particular, the advancements in mRNA technology, which came to prominence during the COVID-19 pandemic, have highlighted the potential of RNA therapeutics to revolutionize the treatment of various diseases, including rare genetic disorders, cancers, and chronic illnesses. With the decreasing costs of sequencing technologies and the growing accessibility of genomic data, researchers and companies in India are increasingly focusing on developing RNA-based therapies tailored to the genetic profiles of Indian patients.
Regional Insights
Based on Region, West India have emerged as the dominating region in the India RNA Therapeutics Market in 2024. West India is emerging as a dominant region in the RNA therapeutics market due to its robust biotechnology and pharmaceutical ecosystem, world-class infrastructure, and supportive government policies. States like Maharashtra and Gujarat are home to some of the largest pharmaceutical hubs in India, with cities like Pune, Mumbai, and Ahmedabad leading in research, manufacturing, and innovation. The presence of established pharmaceutical giants, biotech startups, and research institutions in these areas creates a fertile ground for the development and commercialization of advanced RNA therapeutics.Additionally, West India benefits from a well-developed infrastructure for pharmaceutical manufacturing and exports. Gujarat, in particular, accounts for a significant share of India's pharmaceutical exports and houses advanced facilities for biologics and RNA-based drug production. The region's proximity to major ports further strengthens its export capabilities, enabling faster delivery of RNA-based products to global markets.
Key Market Players
- Pfizer Limited
- Novartis AG
- Sanofi S.A.
- Biocon Ltd Company
- Gennova Biopharmaceuticals Ltd.
- Cipla Limited
- Dr. Reddy's Laboratories
- Sun Pharmaceutical Industries Ltd.
- Bharat Biotech
- Serum Institute of India
Report Scope
In this report, the India RNA Therapeutics Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:India RNA Therapeutics Market, By Product:
- Vaccines
- Drugs
India RNA Therapeutics Market, By Type:
- mRNA Therapeutics
- RNA Interference (RNAi) Therapeutics
- Antisense Oligonucleotide (ASO) Therapeutics
- Others
India RNA Therapeutics Market, By Indication:
- Infectious Diseases
- Rare Genetic Diseases/Hereditary Diseases
- Other
India RNA Therapeutics Market, By End User:
- Hospitals and Clinics
- Research Settings
India RNA Therapeutics Market, By Region:
- East India
- West India
- North India
- South India
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the India RNA Therapeutics Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Pfizer Limited
- Novartis AG
- Sanofi S.A.
- Biocon Ltd Company
- Gennova Biopharmaceuticals Ltd.
- Cipla Limited
- Dr. Reddy's Laboratories
- Sun Pharmaceutical Industries Ltd.
- Bharat Biotech
- Serum Institute of India
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 85 |
| Published | February 2025 |
| Forecast Period | 2024 - 2030 |
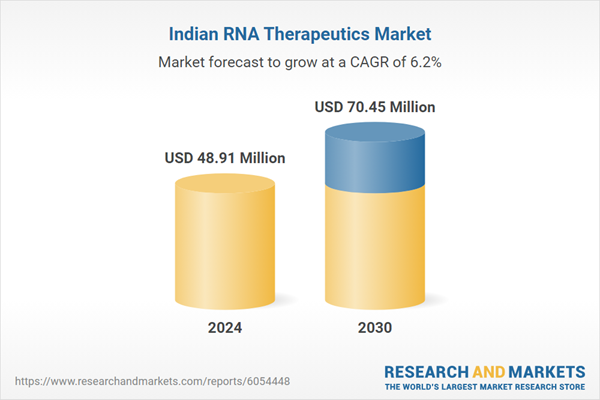
| Estimated Market Value ( USD | $ 48.91 Million |
| Forecasted Market Value ( USD | $ 70.45 Million |
| Compound Annual Growth Rate | 6.2% |
| Regions Covered | India |
| No. of Companies Mentioned | 10 |