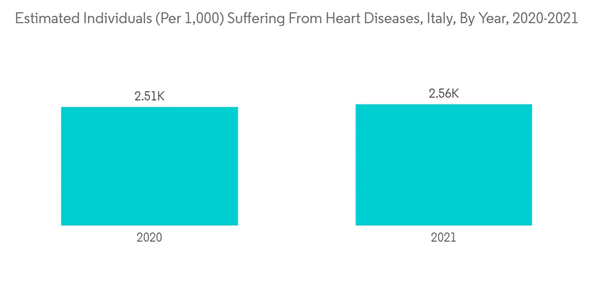
The COVID-19 pandemic has impacted the growth of the Italian cardiovascular devices market. For instance, in January 2021, the National Institute of Health reported that the total number of emergency department (ED) visits for heart issues in Italy was around 2,572 in the year 2020, while in the previous year, the number of ED visits for heart ailments in Italy was around 8,071. A 213.8% reduction in emergency heart visits was observed in Italy during the COVID-19 pandemic, which led to a reduction in demand for cardiovascular devices in Italy. Thus, COVID-19 impacted the growth of the studied market. However, in the current scenario, due to the reduction in COVID-19 cases and the resumption of emergency services in hospitals, the number of patients undergoing cardiac surgeries has increased compared to the beginning of the pandemic, and the demand for cardiovascular devices has increased. Thus, the market is expected to witness significant growth over the forecast period.
The factors driving the growth of the studied market are the increasing burden of cardiovascular diseases coupled with a rising geriatric population, rapid technological advancements, and an increased preference for minimally invasive procedures.
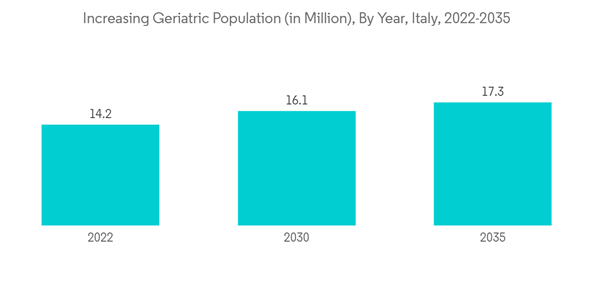
The steadily rising prevalence of chronic heart ailments and sedentary lifestyles engender a high incidence of cardiovascular diseases (CVDs). In addition, the rising geriatric population diagnosed with cardiovascular disorders is contributing to the growth of this market. For instance, in July 2022, the United Nations reported that 14.2 million people aged above 65 years were estimated in Italy, and this number is expected to increase to 18.1 million by the end of 2040. Similarly, an article published by the journal Minerva Cardioangiologica in June 2021 reported the prevalence of CVDs in Italy is expected to increase with aging. Thus, the rising geriatric population is increasing the demand for cardiovascular devices in Italy as this population is more prevalent to CVDs and thus contributing to the growth of the studied market.
Furthermore, the technological advancement leading to new product launches is expected to drive the growth of the studied market. For instance, in October 2022, Thubrikar Aortic Valve, Inc., reported that it had completed the initial CE Mark-enabling study using the Optimum Transcatheter Aortic Valve (Optimum TAV) in patients with severe aortic stenosis. Similarly, in March 2022, Cathi Distribution GmbH launched the Cathis RHC 2 in Europe, including Italy; it is a next-generation suitable heart catheter (RHC) simulator. Thus, such product launches and studies for product approval are likely to contribute to the growth of the studied market.
Thus, with the increasing burden of cardiovascular diseases coupled with a rising geriatric population, rapid technological advancements, and an increased preference for minimally invasive procedures, the market is expected to project growth over the forecast period. However, stringent regulatory policies and high costs associated with cardiovascular devices and procedures are some of the factors hindering market growth in Italy.
Italy Cardiovascular Devices Market Trends
Stents are Expected to Witness Significant Growth Over the Forecast Period.
Coronary stents (CS) are expandable tubular metallic devices that are introduced into coronary arteries that are clogged due to an underlying atherosclerosis disease. Stents are often used to treat narrowed coronary arteries that provide the heart with oxygen-rich blood. Stents are also sometimes used to treat an aneurysm, which is a bulge in the wall of an artery and to treat narrowed airways in the lungs. The rising cases of atherosclerosis disease and new product launches in Italy are expected to drive the growth of the studied market in the country.The rising cases of high cholesterol leading to atherosclerosis disease in Italy are expected to drive the growth of this segment. For instance, an article published by Great Italian Food Trade published in May 2022 reported that an Italian study shows that half of the people with high cholesterol. Most high cholesterol cases may lead to the development of atherosclerosis disease, which may increase the demand for coronary stents, thereby contributing to the growth of this segment.
Additionally, new product launches are expected to drive the growth of the studied market. For instance, in April 2022, Translumina launched the Vivo Isar dual-drug polymer-free coated stent (DES) in Italy and plans to roll it out in several markets across Europe. Thus, such product launches are expected to drive the growth of this segment.
Thus, rising cases of atherosclerosis disease and new product launches in Italy are expected that the segment studied will witness significant growth over the forecast period.
Remote Cardiac Monitoring Segment is Expected to Witness Significant Growth Over the Forecast Period.
Remote cardiac monitoring (RCM), or remote heart monitoring, is a method by which information from a patient's implantable rhythm management device can be communicated directly to a physician's office. The increasing adoption of RCM in Italy, the increasing geriatric population, and technological developments are driving the growth of this segment.The increasing adoption of RCM in recent years in Italy is driving the growth of this segment. For instance, an article published in the journal, The Lancet Digital Health in April 2022 reported the surge in demand for RCM in Italy during 2020. The article quoted that RCM played a significant role in managing patients' cardiac records when hospitals and various healthcare services were closed. Thus, the increasing adoption of cardiac devices to monitor cardiac health is driving the growth of this segment.
Moreover, the increasing geriatric population is more prevalent in various CVDs, and the increasing geriatric population in the country is driving the growth of the studied market in the country. For instance, an article published by the International Journal of Environmental Research and Public Health in August 2022 reported that in Italy, the number of older people aged 65 years and over is about 24% of the total population as of 1st January 2022, and is expected to rise to 30% by 2040. Thus, the increasing geriatric population is more prevalent in various CVDs, which is increasing the demand for RCM devices and contributing to this segment's growth.
Furthermore, collaborations and new product launches are driving the growth of the studied market. For instance, in March 2022, Biotronik received the CE approval for HeartInsight, its remote heart failure (HF) management device that accurately identifies patients at higher risk of HF decompensation early on. Compared to other available solutions, HeartInsight provides the earliest notification, median advance notice of 42 days before an impending HF hospitalization, which allows clinicians to proactively care for their patients. Thus, such device launches are driving the growth of this segment. Thus, due to the increasing adoption of RCM in Italy, the increasing geriatric population and new product launches are driving the growth of this segment over the forecast period.
Italy Cardiovascular Devices Industry Overview
The Italy Cardiovascular Devices Market is moderately competitive, with a few local manufacturers competing with international companies. Some of the companies working in this market are Abbott Laboratories, Boston Scientific Corporation, Cardinal Health Inc., Edwards Lifesciences Corp, GE Healthcare, WL Gore & Associates Inc., Medtronic PLC, MS Holding SE & Co. KG (Biotronik SE & Co. KG), Siemens Healthineers AG and Canon Medical Systems Corporation.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Boston Scientific Corporation
- Cardinal Health Inc.
- Edwards Lifesciences Corp
- GE Healthcare
- WL Gore & Associates Inc.
- Medtronic PLC
- MS Holding SE & Co. KG (Biotronik SE & Co. KG)
- Siemens Healthineers AG
- Canon Medical Systems Corporation