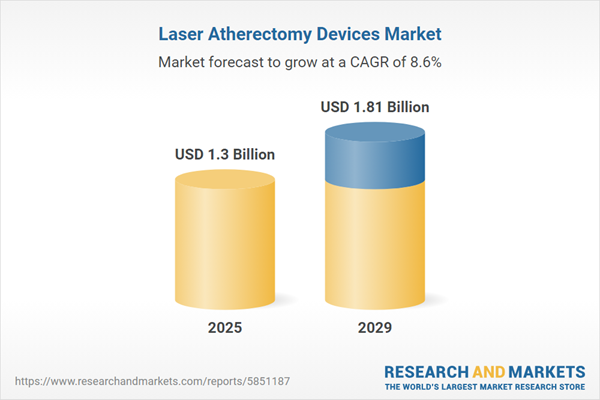
The laser atherectomy devices market size is expected to see strong growth in the next few years. It will grow to $1.81 billion in 2029 at a compound annual growth rate (CAGR) of 8.6%. The growth in the forecast period can be attributed to demand for minimally invasive procedures, rise in cardiovascular conditions, expansion of regulatory approvals, advancements in device safety and efficiency, physician training programs. Major trends in the forecast period include patient-centric care, miniaturization and device portability, personalized treatment approaches, integration of imaging technologies, focus on safety and efficacy.
An increase in the prevalence of atherosclerosis is anticipated to drive the growth of the laser atherectomy devices market. Atherosclerosis is a medical condition characterized by the accumulation of fat within the arteries, leading to their narrowing and hardening. This can impede blood flow to essential organs and tissues, resulting in severe health complications such as heart disease, stroke, and peripheral artery disease. Laser atherectomy devices are employed in the treatment of atherosclerosis by utilizing a laser to vaporize the plaque, which is subsequently removed from the body via a catheter. For example, in March 2023, the Sentinel Stroke National Audit Programme, a UK-based initiative focused on healthcare quality improvement, reported that there were 91,162 patients hospitalized for stroke in 2022/23, a slight decrease from 92,054 in 2021/22. Thus, the rising prevalence of atherosclerosis is a key factor driving the growth of the laser atherectomy devices market.
The laser atherectomy devices market is also anticipated to witness growth due to the rising incidence of cardiovascular diseases (CVD). CVD encompasses diseases affecting the heart or blood vessels, impacting the circulatory system. The surge in cardiovascular diseases leads to an increased number of patients requiring treatment for arterial occlusions and atherosclerotic plaque, thereby creating a higher demand for laser atherectomy devices. In August 2022, the American College of Cardiology projected an increase in all four cardiovascular risk factors between 2025 and 2060 in the United States, with diabetes, dyslipidemia, hypertension, and obesity on the rise. The report highlighted substantial projected increases in stroke, heart failure, ischemic heart disease, and heart attack rates. Therefore, the growth of cardiovascular diseases propels the laser atherectomy devices market.
Leading companies in the laser atherectomy devices market are actively innovating to create advanced products, including atherectomy systems, to cater to broader customer bases, drive increased sales, and boost overall revenue. An atherectomy system, a medical device specifically crafted for the removal of atherosclerotic plaque from blood vessels, exemplifies this trend. For instance, in August 2022, AngioDynamics Inc., a US-based medical device company, revealed that the Auryon Atherectomy System had received Expanded Indications clearance from the United States Food and Drug Administration (FDA). The notable feature of the Auryon Atherectomy System lies in its capacity to effectively address various infrainguinal lesion types, spanning above-the-knee (ATK), below-the-knee (BTK), and in-stent restenosis (ISR). Employing cutting-edge technology, this system delivers powerful treatment for arterial occlusions. Significantly, it distinguishes itself as a pioneering laser atherectomy system capable of efficiently treating lesions of any type, length, and location with minimal impact on vessel walls.
Prominent companies in the laser atherectomy devices market are also investing efforts in developing advanced solutions, such as atherectomy platforms, to meet the demands of larger customer bases, drive more sales, and enhance revenue. An atherectomy platform constitutes a comprehensive system or set of tools designed for treating atherosclerosis - a medical condition characterized by plaque buildup in arteries. For instance, in October 2023, Cardio Flow Inc., a US-based medical device company, announced FDA clearance for the FreedomFlow, an orbital atherectomy peripheral platform. The distinctive feature of the FreedomFlow platform lies in its contemporary mechanism of action tailored to clear plaque blockages in leg arteries. Its exclusive catheter-based design leverages angular momentum physics, creating a spiral geometry that ensures simultaneous contact with the vessel wall during both advancement and retraction through the incorporation of five diamond-coated spheres. Additionally, a diamond-coated tip facilitates the smooth passage of the driveshaft through narrow blockages. This innovative approach provides physicians with an exceptionally efficient, effective, and adaptable means of treating complex Peripheral Artery Disease (PAD) across a broad spectrum of vessel diameters, ranging from 2 mm in the ankle to 8 mm in the hip. The platform offers greater versatility in addressing multiple arteries and multiple blockages within the same vessel, all achieved with a single device.
In April 2023, Abbott Laboratories, a US-based medical device company, completed the acquisition of Cardiovascular Systems, Inc. for an undisclosed amount. This strategic move enhances Abbott's capabilities to address vascular disease by incorporating CSI's leading atherectomy system designed to prepare vessels for angioplasty or stenting, ultimately restoring blood flow. The acquisition aligns with Abbott's broader investment strategy in its vascular portfolio, fortifying its ability to deliver comprehensive care for patients dealing with peripheral and coronary artery disease. Cardiovascular Systems Inc., a US-based manufacturer of atherectomy devices, now operates as part of Abbott's portfolio.
Major companies operating in the laser atherectomy devices market include Boston Scientific Corporation, Terumo Corporation, Medtronic plc, Avinger Inc., Cardiovascular Systems Inc., Koninklijke Philips N.V., AngioDynamics Inc., Becton, Dickinson and Company, Eximo Medical Ltd., Cardinal Health, B. Braun SE, C. R. Bard Inc., Abbott Laboratories, Biotronik SE & Co. KG, Rex Medical, Spectranetics Corporation, Straub Medical AG, Vascular Solutions Inc., Volcano Corporation, Atrium Medical Corporation, Bayer AG, Biosensors International Group Ltd., Cook Medical LLC, Cordis Corporation, Covidien plc, Medtronic Vascular, Shockwave Medical Inc., Acotec Scientific Holdings Ltd., Advanced Catheter Therapies Inc., ArioMedica Ltd.
North America was the largest region in the laser arthectomy devices market in 2024. Asia-Pacific is expected to be the fastest-growing region in the global laser atherectomy devices market report during the forecast period. The regions covered in the laser atherectomy devices market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the laser atherectomy devices market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
A laser atherectomy device is a medical instrument designed to employ laser energy for breaking down and removing blockages from blood vessels. This device is commonly utilized in the treatment of conditions such as peripheral artery disease (PAD) or atherosclerosis, where plaque buildup in the arteries leads to narrowing or blockages. The laser atherectomy device operates by delivering a high-energy laser beam to the plaque, causing it to vaporize and facilitating the removal of the blockage.
The primary types of laser atherectomy devices include those with computer control and others. Computer control involves the utilization of computer technology to manage and control various systems and processes. These devices find applications in cardiovascular, peripheral vascular, and neurovascular procedures and are employed by hospitals and surgical centers, ambulatory care centers, and other medical facilities.
The laser atherectomy devices market research report is one of a series of new reports that provides laser atherectomy devices market statistics, including laser atherectomy devices industry global market size, regional shares, competitors with a laser atherectomy devices market share, detailed laser atherectomy devices market segments, market trends and opportunities, and any further data you may need to thrive in the laser atherectomy devices industry. This laser atherectomy devices market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The laser atherectomy devices market consists of sales of turbo elite, diamondback 360, phoenix atherectomy system, jetstream atherectomy system, hawkone system, and ocelot system. Values in this market are ‘factory gate’ values, that is, the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Laser Atherectomy Devices Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on laser atherectomy devices market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for laser atherectomy devices? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The laser atherectomy devices market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Type: Computer Control; Other Types2) By Application: Cardiovascular; Peripheral Vascular; Neurovascular
3) By End-User: Hospitals and Surgical Centers; Ambulatory Care Centers; Other End-Users
Subsegments:
1) By Computer Control: Automated Laser Atherectomy Systems; Computerized Navigation Systems2) By Other Types: Manual Laser Atherectomy Devices; Portable Laser Atherectomy Devices
Key Companies Mentioned: Boston Scientific Corporation; Terumo Corporation; Medtronic plc; Avinger Inc.; Cardiovascular Systems Inc.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
- Boston Scientific Corporation
- Terumo Corporation
- Medtronic plc
- Avinger Inc.
- Cardiovascular Systems Inc.
- Koninklijke Philips N.V.
- AngioDynamics Inc.
- Becton, Dickinson and Company
- Eximo Medical Ltd.
- Cardinal Health
- B. Braun SE
- C. R. Bard Inc.
- Abbott Laboratories
- Biotronik SE & Co. KG
- Rex Medical
- Spectranetics Corporation
- Straub Medical AG
- Vascular Solutions Inc.
- Volcano Corporation
- Atrium Medical Corporation
- Bayer AG
- Biosensors International Group Ltd.
- Cook Medical LLC
- Cordis Corporation
- Covidien plc
- Medtronic Vascular
- Shockwave Medical Inc.
- Acotec Scientific Holdings Ltd.
- Advanced Catheter Therapies Inc.
- ArioMedica Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | February 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 1.3 Billion |
| Forecasted Market Value ( USD | $ 1.81 Billion |
| Compound Annual Growth Rate | 8.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 30 |