Increasing Outsourcing of R&D Activities by Pharmaceutical Companies fuel the North America Bioanalytical Testing Services Market
Pharmaceutical companies outsource research & development activities that are not core to their internal structure. Outsourcing miscellaneous activities allows them to efficiently focus on their internal core competencies for making the drug development process better and more cost effective. Moreover, outsourcing manufacturing activities, along with R&D, benefits them by lowering the turnaround period, adding to their expertise, and eliminating the need for large capital investments. According to the Survey of Biopharmaceutical Manufacturing Capacity and Production by BioPlan Associates, analytical testing/bioassay was the most outsourced service in 2022, followed by toxicity testing, validation services, product characterization, and others. While developing nations are the largest contributors to the demand for bioanalytical testing services through contract manufacturing, the US remains a potential outsourcing destination. 39.6% of non-US respondents in the survey preferred outsourcing to US-based facilities. Thus, the practice of outsourcing is gaining popularity worldwide, especially in developing countries, thereby favoring the growth of the bioanalytical testing services market. Pharmaceutical businesses are widely implementing the quality-by-design (QbD) concept, which is further propelling the adoption of outsourcing services by pharmaceutical companies to increase the robustness of their production processes and ensure optimal product quality and manufacturing productivity. QbD is supported by regulatory bodies such as the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA). The concept has gained traction in the pharmaceutical industry over the years with the publication of ICH Q9 (Quality Risk Management), ICH Q8 (R2, Pharmaceutical Development), and ICH Q10 (Pharmaceutical Quality System). According to the ICH Q10 guidelines, analytical methods are essential to the pharmaceutical quality system. Analytical QbD (AQbD) implementation in manufacturing ensures product quality and performance. Outsourcing bioanalytical testing services helps pharmaceutical businesses reduce business risks by avoiding major investments in analytical equipment and skilled professionals, especially while the product is in the early phase of development. Due to the availability of specialized analytical testing service providers with crucial competencies to quickly provide excellent results, pharmaceutical companies are increasingly considering outsourcing bioanalytical testing services to third-party service providers, which fuels the market growth.North America Bioanalytical Testing Services Market Overview
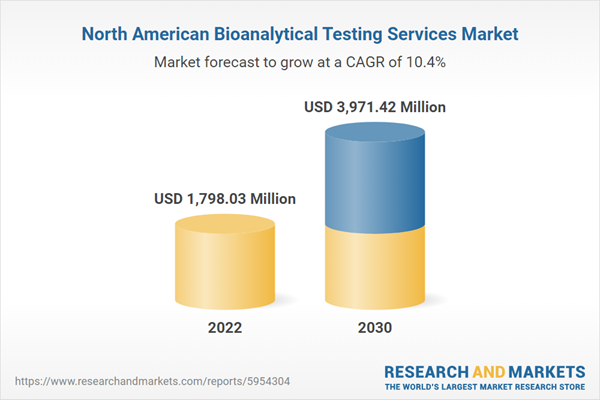
North America holds the largest share of the bioanalytical testing services market, with the US, Canada, and Mexico being the major contributors to the market growth in this region. The uninterrupted growth of the pharmaceuticals industry, the increasing need for developing novel drugs due to an upsurge in the incidences of various diseases, rising healthcare expenditure by governments, and a growing preference for outsourcing activities for the quality assurance of pharmaceutical products are the factors favoring the growth of the bioanalytical testing services market in North America.Nort
h America Bioanalytical Testing Services Market Revenue and Forecast to 2030 (US$ Million)
North America Bioanalytical Testing Services Market Segmentation
The North America bioanalytical testing services market is segmented based on test type, disease indication, end user, and country.Based on test type, the North America bioanalytical testing services market is segmented into pharmacokinetics, biomarkers, immunogenicity, virology testing, cell-based assays, and others. The cell-based assays segment held the largest market share in 2022.
Based on disease indication, the North America bioanalytical testing services market is segmented into cardiovascular, neurological disorders, metabolic disorders, respiratory diseases, autoimmune disorder, oncology, sexual health, bone disease, and others. The oncology segment held the largest market share in 2022.
Based on end user, the North America bioanalytical testing services market is segmented into pharmaceutical and biopharmaceutical companies, contract research organization (CRO), contract development and manufacturing organization (CDMO), and others. The pharmaceutical and biopharmaceutical companies segment held the largest market share in 2022.
Based on country, the North America bioanalytical testing services market is segmented into the US, Canada, and Mexico. The US dominated the North America bioanalytical testing services market share in 2022.
SGS SA, Pharmaron Beijing Co Ltd, Element Materials Technology Group Ltd, CD BioSciences Inc, Charles River Laboratories International Inc, Eurofins Scientific SE, Labcorp Drug Development Inc, Syneos Health Inc, KCAS Bioanalytical and Biomarker Services LLC, ICON Plc, and Intertek Group Plc are some of the leading companies operating in the North America bioanalytical testing services market.
Reasons to Buy
- Save and reduce time carrying out entry-level research by identifying the growth, size, leading players, and segments in the North America bioanalytical testing services market.
- Highlights key business priorities in order to assist companies to realign their business strategies
- The key findings and recommendations highlight crucial progressive industry trends in the North America bioanalytical testing services market, thereby allowing players across the value chain to develop effective long-term strategies
- Develop/modify business expansion plans by using substantial growth offering developed and emerging markets
- Scrutinize in-depth North America market trends and outlook coupled with the factors driving the North America bioanalytical testing services market, as well as those hindering it
- Enhance the decision-making process by understanding the strategies that underpin commercial interest with respect to client products, segmentation, pricing, and distribution
Table of Contents
Companies Mentioned
- SGS SA
- Pharmaron Beijing Co Ltd
- Element Materials Technology Group Ltd
- CD BioSciences Inc
- Charles River Laboratories International Inc
- Eurofins Scientific SE
- Labcorp Drug Development Inc
- Syneos Health Inc
- KCAS Bioanalytical and Biomarker Services LLC
- ICON Plc,
- Intertek Group Plc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 110 |
| Published | February 2024 |
| Forecast Period | 2022 - 2030 |
| Estimated Market Value ( USD | $ 1798.03 Million |
| Forecasted Market Value ( USD | $ 3971.42 Million |
| Compound Annual Growth Rate | 10.4% |
| Regions Covered | North America |
| No. of Companies Mentioned | 11 |