In North America, till April 2022, the United States is having the highest COVID cases with 82 million, the country also registered the highest death rate. According to the Diabetes Voice article published in May 2020, close to 40,000 deaths of people who are having diabetes. In the North American region diabetes patients are more concerned about stocking up the monitoring and managing devices due to this the market increased during these years.
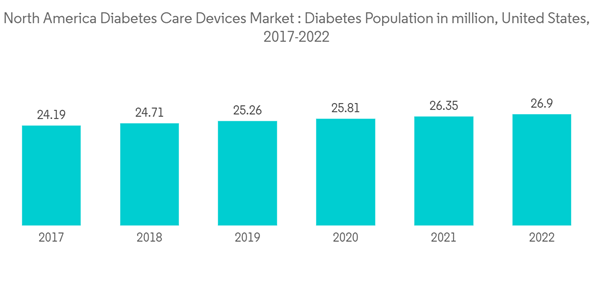
The North American region had witnessed an astounding increase in the prevalence of diabetes, in recent years. In developed countries, such as the United States and Canada, the rate of diabetes is at an all-time high, mainly due to lifestyle changes. Diabetes is associated with many health complications. Comparing the population with and without diabetes, those with diabetes have a 300% increased risk of being hospitalized and thus incur more healthcare expenses compared to non-diabetic people. Patients with Type 2 diabetes require many corrections throughout the day for maintaining nominal blood glucose levels, such as the administration of additional insulin or ingestion of additional carbohydrates. Furthermore, patients attempting to control their blood glucose levels tightly to prevent the long-term complications associated with fluctuations in blood glucose levels are at greater risk for overcorrection and the resultant hypoglycemia. Achieving nominal results can be very difficult without multiple daily injections of insulin or insulin pump therapy. This is driving the demand for diabetes care devices in North America, thereby driving the market in focus during the forecast period.
North America Diabetes Care Devices Market Trends
Monitoring Devices is Having the Highest Market Share in Current Year
The monitoring devices segment is expected to increase with a CAGR of over 7% during the forecast period, mainly due to the demand from the type-1 diabetes population, which is expected to be more than 60 million by the end of 2028.Monitoring devices are garnering widespread adoption due to the availability of reimbursement options for glucose meters. Glucose meter devices must be replaced within six to eight months and are very expensive. As a result, most people prefer selecting health insurance plans that cover almost 80% of the total expenditure on healthcare devices. Such schemes cover the cost of diabetes testing supplies, blood glucose test strips, and blood glucose meters. For example, Medicare, the federal health insurance program in the United States, covers approximately 80% of the cost of BGM devices for diabetic people. The usage of CGM devices is on the rise in North America because of their advancement in technology and their ability to empower people with diabetes to make more informed decisions about their health. This is driving the demand for diabetes care devices in North America, thereby driving the market in focus during the forecast period.
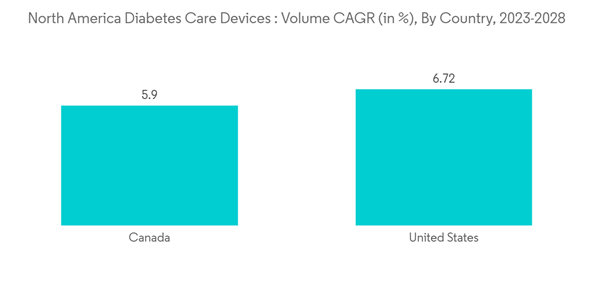
The United States is Expected to Dominate the North America Diabetes Care Devices Market.
The United States is expected to grow tremendously during the forecast period, owing to factors such as the high prevalence of obesity and increasing awareness regarding diabetes care in the region.According to the American Diabetes Association, 1.4 million Americans are thought to receive a diabetes diagnosis each year. In Canada and other North American nations, diabetes is one of the main killers. The disease's rising incidence, prevalence, and progressive nature have motivated the development of novel medications to give diabetic patients more treatment options. Non-insulin medications, which serve as first-line therapies for people with type-2 diabetes, currently account for more than half of sales in the anti-diabetic market.
Innovations in insulin pump devices in the United States are driving the market. In July 2021, Medtronic received FDA clearance for its new extended-wear infusion set, designed to last more than twice as long as existing infusion sets that connect traditional tubed pumps to the body for insulin delivery. That means it can be worn on the body for up to 7 days, compared to existing sets that must be changed every 2 or 3 days. In the United States, there are innovations from start-up companies with approximately USD 2.9 billion in funding in the diabetes industry, like Glooko, OneDrop, Verily, Vacate, Insulet, Noom, Bigfoot Biomedical, Virta Health, Diabeloop, Orgenesis, etc. Thus, owing to the above factors, it is expected to drive market growth over the forecast period.
North America Diabetes Care Devices Industry Overview
A select few big players control the market. Abbott's new FreeStyle Libre Flash glucose monitoring system was approved, which is the first continuous blood sugar monitor for people with diabetes that does not need backup finger prick tests. Innovations in test strips are increasing and are under development. For example, the new test strip includes features such as underfill detection and provides the user with the ability to reapply blood when the test strip is underfilled ("second chance" sampling). Dexcom acquired TypeZero Technologies, paving the way for automated insulin delivery. The acquisition has sent Dexcom ahead in the race to create an artificial pancreas system rather than simply offering a continuous glucose monitoring device.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Becton Dickinson and Company
- Medtronic
- F. Hoffmann-La Roche AG
- Insulet
- Abbott
- Dexcom
- Tandem
- Ypsomed
- Novo Nordisk
- Sanofi
- Eli Lilly