The US market dominated the North America Laboratory Developed Tests Market by Country in 2023, and would continue to be a dominant market till 2031; thereby, achieving a market value of$5.62 billion by 2031. The Canada market is experiencing a CAGR of 6.9% during (2024 - 2031). Additionally, The Mexico market would exhibit a CAGR of 7.6% during (2024 - 2031).
LDTs find applications across various healthcare domains, spanning diagnostic testing, disease monitoring, therapeutic decision-making, and research. These tests are utilized in diverse clinical settings, including hospital laboratories, reference laboratories, academic research centers, and specialized diagnostic facilities. LDTs are used for the diagnosis of a wide range of diseases and conditions, including infectious diseases, cancer, cardiovascular disorders, autoimmune disorders, and genetic disorders.
In addition, LDTs play a critical role in patient stratification and personalized medicine by identifying molecular subtypes, treatment targets, and predictive biomarkers associated with differential treatment responses or disease outcomes. These tests facilitate selecting optimal treatment strategies tailored to individual patient characteristics, genetic profiles, and clinical contexts.
As healthcare expenditure increases in Canada, there is a corresponding rise in demand for diagnostic testing across various medical specialties and disease areas. According to the Canadian government, the provinces and territories will get an additional $46.2 billion in financing as part of a $196.1 billion ten-year investment by the government to enhance health care services for all Canadians. This brings the total amount of money it will spend over ten years from $198.6 billion to $12.5 billion for supporting indigenous goals and supplementary government support. Likewise, as cancer rates rise in Mexico, there is a growing demand for screening and diagnostic tests to detect cancer at early stages when treatment is most effective. Mexico’s third most common cause of death, according to All.Can, is cancer. In 2020, around 195,000 new cases were reported. The cancers with the highest prevalence were cervical (9,439), thyroid (11,227), colon (11,191), prostate (26.742), breast (29.929), and thyroid (11,227). Thus, rising healthcare spending and high cancer rates propel the market’s expansion.
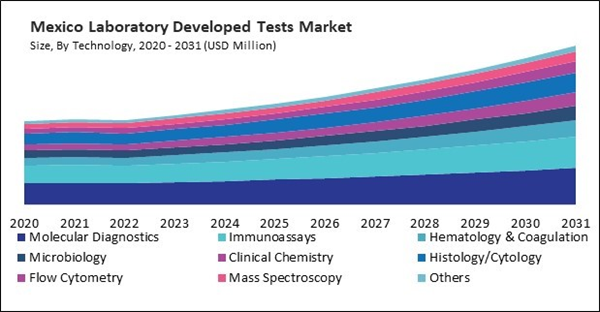
Based on Application, the market is segmented into Oncology, Genetic Disorders/Inherited Disease, Infectious & Parasitic Diseases, Endocrine, Immunology, Nutritional & Metabolic Disease, Cardiology, Mental/Behavioral Disorder, Pediatrics-specific Testing, and Others. Based on Technology, the market is segmented into Molecular Diagnostics, Immunoassays, Hematology & Coagulation, Microbiology, Clinical Chemistry, Histology/Cytology, Flow Cytometry, Mass Spectroscopy, and Others. Based on countries, the market is segmented into U.S., Mexico, Canada, and Rest of North America.
List of Key Companies Profiled
- Neogenomics, Inc.
- Guardant Health, Inc.
- Qiagen N.V
- Quest Diagnostics Incorporated
- Abbott Laboratories
- Siemens Healthineers AG (Siemens AG)
- Illumina, Inc.
- Bio-Rad Laboratories, Inc.
- F.Hoffmann-La Roche Ltd.
- Eurofins Scientific SE
Market Report Segmentation
By Application- Oncology
- Genetic Disorders/Inherited Disease
- Infectious & Parasitic Diseases
- Endocrine
- Immunology
- Nutritional & Metabolic Disease
- Cardiology
- Mental/Behavioral Disorder
- Pediatrics-specific Testing
- Others
- Molecular Diagnostics
- Immunoassays
- Hematology & Coagulation
- Microbiology
- Clinical Chemistry
- Histology/Cytology
- Flow Cytometry
- Mass Spectroscopy
- Others
- US
- Canada
- Mexico
- Rest of North America
Table of Contents
Companies Mentioned
- Neogenomics, Inc.
- Guardant Health, Inc.
- Qiagen N.V
- Quest Diagnostics Incorporated
- Abbott Laboratories
- Siemens Healthineers AG (Siemens AG)
- Illumina, Inc.
- Bio-Rad Laboratories, Inc.
- F. Hoffmann-La Roche Ltd.
- Eurofins Scientific SE