The US market dominated the North America Pharmaceutical Sterility Testing Market by Country in 2022 and would continue to be a dominant market till 2030; thereby, achieving a market value of $1,038.4 Million by 2030. The Canada market is experiencing a CAGR of 12.7% during (2023 - 2030). Additionally, The Mexico market would exhibit a CAGR of 12.3% during (2023 - 2030).
The pharmaceutical sterility testing market plays a pivotal role in ensuring the safety and efficacy of pharmaceutical products, with a primary focus on preventing microbial contamination. As an integral component of quality control within the pharmaceutical industry, sterility testing is designed to detect the presence of viable microorganisms in drug products, ensuring that they meet the stringent regulatory standards set by health authorities.
Additionally, sterility testing applications span various stages of the pharmaceutical manufacturing process, covering many products, including injectables, ophthalmic solutions, vaccines, and parenteral drugs. The testing is conducted on final drug products and raw materials to identify and eliminate potential sources of contamination. The growing drug demand necessitates an increase in sterility testing.
The United States Food and Drug Administration (FDA) imposes strict regulatory requirements on pharmaceuticals and medical devices. Sterility testing is a fundamental aspect of ensuring compliance with these regulations. As the medical device sector expands, the need for rigorous sterility testing grows parallel to meet regulatory standards. The U.S. medical device sector covers many products, including implantable devices, diagnostic equipment, and in vitro diagnostic devices. Hence, North America's rising medical device sector is expected to boost the region's demand for pharmaceutical sterility testing.
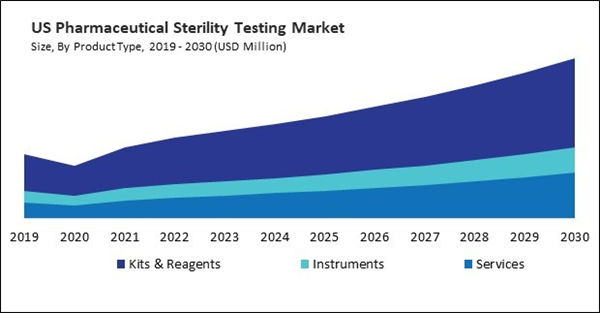
Based on Type, the market is segmented into Outsourcing, and In-House. Based on Product Type, the market is segmented into Kits & Reagents, Instruments, and Services. Based on Sample, the market is segmented into Pharmaceuticals, Medical Devices, and Biopharmaceuticals. Based on End-use, the market is segmented into Pharmaceutical Companies, Medical Device Companies, Compounding Pharmacies, and Others. Based on Test Type, the market is segmented into Bioburden Testing, Sterility Testing, and Bacterial Endotoxin Testing. Based on countries, the market is segmented into U.S., Mexico, Canada, and Rest of North America.
List of Key Companies Profiled
- Steris PLC
- Charles River Laboratories International, Inc.
- Thermo Fisher Scientific, Inc.
- SGS S.A.
- Sartorius AG
- Sotera Health Company
- Pacific Biolabs, Inc.
- Laboratory Corporation of America Holdings
- Almac Group
- Pace Analytical Services, LLC
Market Report Segmentation
By Type- Outsourcing
- In-House
- Kits & Reagents
- Instruments
- Services
- Pharmaceuticals
- Medical Devices
- Biopharmaceuticals
- Pharmaceutical Companies
- Medical Device Companies
- Compounding Pharmacies
- Others
- Bioburden Testing
- Sterility Testing
- Bacterial Endotoxin Testing
- US
- Canada
- Mexico
- Rest of North America
Table of Contents
Companies Mentioned
- Steris PLC
- Charles River Laboratories International, Inc.
- Thermo Fisher Scientific, Inc.
- SGS S.A.
- Sartorius AG
- Sotera Health Company
- Pacific Biolabs, Inc.
- Laboratory Corporation of America Holdings
- Almac Group
- Pace Analytical Services, LLC