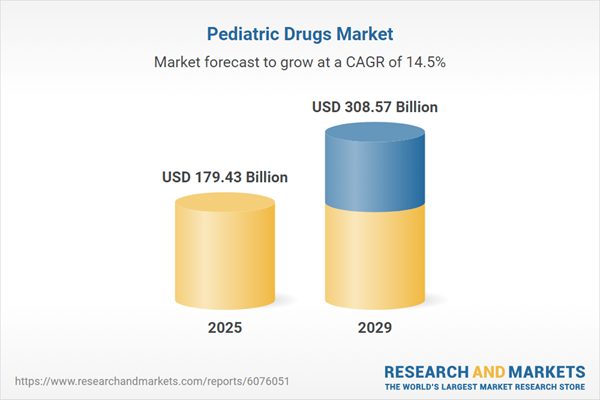
The pediatric drugs market size is expected to see rapid growth in the next few years. It will grow to $308.57 billion in 2029 at a compound annual growth rate (CAGR) of 14.5%. The growth in the forecast period can be attributed to increasing pediatric population, rising prevalence of pediatric mental health issues, rising number of children with chronic conditions, higher vaccination rate in children and rising demand for pediatric mental health medications. Major trends in the forecast period include personalized pediatric medicine, technological integration in pediatric drug delivery, telemedicine and digital health solutions, enhance pediatric clinical trials, and collaboration between pediatricians and pharmacists.
The forecast of 14.5% growth over the next five years reflects a modest reduction of 0.4% from the previous estimate for this market. This reduction is primarily due to the impact of tariffs between the US and other countries. Trade tensions could hinder U.S. children's hospitals by inflating prices of specialized pediatric formulations manufactured in Sweden and the Netherlands, resulting in delayed treatment for chronic childhood conditions and raising pediatric care costs. The effect will also be felt more widely due to reciprocal tariffs and the negative effect on the global economy and trade due to increased trade tensions and restrictions.
The increasing number of preterm births is anticipated to drive the growth of the pediatric drugs market in the coming years. Preterm births refer to infants born before completing 37 weeks of gestation, which is earlier than the typical 40-week term. Several factors contribute to the rise in preterm births, including increased maternal age, lifestyle choices, medical conditions, multiple pregnancies, and inadequate prenatal care. Pediatric drugs play a crucial role in supporting the health and development of preterm infants by addressing challenges such as respiratory complications, infections, and nutritional deficiencies. For example, in December 2022, a report published by Tommy’s, a UK-based charity organization, indicated that the percentage of premature live births in England and Wales increased to 7.9% in 2022, up from 7.5% in 2021. This rise accounted for more than 53,000 babies born prematurely in 2022. As a result, the growing number of preterm births is fueling the expansion of the pediatric drugs market.
Leading companies in the pediatric drugs market are focusing on developing innovative treatments, such as fully human monoclonal antibodies, to enhance therapeutic outcomes and reduce side effects for young patients. A fully human monoclonal antibody (mAb) is derived entirely from human genetic sequences, minimizing the risk of immune reactions compared to chimeric or humanized monoclonal antibodies. These antibodies are produced using advanced biotechnological techniques, such as transgenic mice with human antibody genes or phage display technology. For instance, in August 2022, Johnson & Johnson, a US-based pharmaceutical company, received FDA approval for STELARA (ustekinumab) to treat pediatric patients aged six years and older with active psoriatic arthritis (PsA). This approval marked a significant breakthrough, as STELARA is the first and only biologic therapy targeting interleukin-12 (IL-12) and interleukin-23 (IL-23), cytokines that play a key role in the inflammatory responses associated with autoimmune diseases. Active PsA is a rare condition that affects approximately 5-8% of children with chronic inflammatory arthritis, causing symptoms such as joint swelling and skin lesions.
In July 2022, JB Chemicals & Pharma Limited, an India-based pharmaceutical company, acquired four pediatric brands from Dr. Reddy's Laboratories for ₹98.3 crore (approximately $1.18 million). This acquisition was aimed at expanding JB Pharma’s presence in the pediatric segment by leveraging the market leadership of the acquired brands to strengthen its portfolio and support future growth ambitions in India. Dr. Reddy's Laboratories Limited is an India-based pharmaceutical company specializing in pediatric drug development.
Major players in the pediatric drugs market are Pfizer Inc., Johnson & Johnson, Merck & Co. Inc., Bayer AG, Sanofi S.A., Bristol-Myers Squibb, AstraZeneca Plc, Novartis AG, GlaxoSmithKline plc, Takeda Pharmaceutical Company, Eli Lilly and Co., Gilead Sciences Inc., Amgen Inc., Teva Pharmaceutical Industries Ltd., Otsuka Pharmaceutical Co. Ltd., Vertex Pharmaceuticals, UCB Pharma, Sun Pharmaceutical Industries Ltd., Perrigo Company, Shionogi & Co. Ltd., Mallinckrodt, Torrent Pharmaceuticals Ltd, Sarepta Therapeutics, Zydus Cadila.
North America was the largest region in the pediatric drugs market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in pediatric drugs report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the pediatric drugs market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
The pediatric drugs market consists of sales of antibiotics, vaccines, pediatric pain relief, respiratory medications, antihistamines, and pediatric dermatology products. Values in this market are ‘factory gate’ values, that is, the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the resulting trade tensions in spring 2025 are having a significant impact on the pharmaceutical sector. Companies are grappling with higher costs on imported active pharmaceutical ingredients (APIs), glass vials, and laboratory equipment - many of which have limited alternative sources. Generic drug manufacturers, already operating with minimal profit margins, are particularly affected, with some scaling back production of low-margin medications. Biotech firms are also experiencing delays in clinical trials due to shortages of specialized reagents linked to tariffs. In response, the industry is shifting API production to regions like India and Europe, building up inventory reserves, and advocating for tariff exemptions on essential medicines.
The pediatric drugs market research report is one of a series of new reports that provides pediatric drugs market statistics, including the pediatric drugs industry global market size, regional shares, competitors with the pediatric drugs market share, detailed pediatric drugs market segments, market trends, and opportunities, and any further data you may need to thrive in the pediatric drugs industry. This pediatric drug market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
Pediatric drugs are medications specifically formulated, dosed, and approved for use in infants, children, and adolescents. These drugs are designed to treat a wide range of medical conditions in pediatric patients, taking into account their unique physiological and metabolic differences from adults.
The primary types of pediatric drugs include respiratory disorder drugs, autoimmune disorder drugs, gastrointestinal drugs, and cardiovascular drugs. Respiratory disorder drugs are formulated to manage breathing-related conditions in children, such as asthma, cystic fibrosis, and respiratory syncytial virus (RSV). These medications can be administered through various routes, including oral, topical, and parenteral methods. They are distributed through multiple channels, such as hospital pharmacies, retail pharmacies, and online pharmacies. Pediatric drugs are utilized by various end users, including hospitals, specialty clinics, homecare settings, and other healthcare facilities.
The market value is defined as the revenues that enterprises gain from the sale of goods and services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 1-3 business days.
Table of Contents
Executive Summary
Pediatric Drugs Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on pediatric drugs market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for pediatric drugs? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The pediatric drugs market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Type: Respiratory Disorder Drugs; Autoimmune Disorder Drugs; Gastrointestinal Drugs; Cardiovascular Drugs2) By Route of Administration: Oral; Topical; Parenteral; Other Route of Administrations
3) By Distribution Channel: Hospital Pharmacy; Retail Pharmacy; Online Pharmacy; Other Distribution Channels
4) By End-Users: Hospitals; Specialty Clinics; Homecare; Other End-Users
Subsegments:
1) By Respiratory Disorder Drugs: Asthma Medications; Cystic Fibrosis Treatments; Bronchodilators; Corticosteroids2) By Autoimmune Disorder Drugs: Immunosuppressants; Biologic Therapies; Disease-Modifying Antirheumatic Drugs (DMARDs)
3) By Gastrointestinal Drugs: Proton Pump Inhibitors (PPIs); Antiemetics; Antidiarrheals; Laxatives
4) By Cardiovascular Drugs: Antihypertensive Medications; Heart Failure Treatments; Antiarrhythmic Drugs; Lipid-lowering Agents
Companies Mentioned: Pfizer Inc.; Johnson & Johnson; Merck & Co. Inc.; Bayer AG; Sanofi S.A.; Bristol-Myers Squibb; AstraZeneca Plc; Novartis AG; GlaxoSmithKline plc; Takeda Pharmaceutical Company; Eli Lilly and Co.; Gilead Sciences Inc.; Amgen Inc.; Teva Pharmaceutical Industries Ltd.; Otsuka Pharmaceutical Co. Ltd.; Vertex Pharmaceuticals; UCB Pharma; Sun Pharmaceutical Industries Ltd.; Perrigo Company; Shionogi & Co. Ltd.; Mallinckrodt; Torrent Pharmaceuticals Ltd; Sarepta Therapeutics; Zydus Cadila
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Pediatric Drugs market report include:- Pfizer Inc.
- Johnson & Johnson
- Merck & Co. Inc.
- Bayer AG
- Sanofi S.A.
- Bristol-Myers Squibb
- AstraZeneca Plc
- Novartis AG
- GlaxoSmithKline plc
- Takeda Pharmaceutical Company
- Eli Lilly and Co.
- Gilead Sciences Inc.
- Amgen Inc.
- Teva Pharmaceutical Industries Ltd.
- Otsuka Pharmaceutical Co. Ltd.
- Vertex Pharmaceuticals
- UCB Pharma
- Sun Pharmaceutical Industries Ltd.
- Perrigo Company
- Shionogi & Co. Ltd.
- Mallinckrodt
- Torrent Pharmaceuticals Ltd
- Sarepta Therapeutics
- Zydus Cadila
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | September 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 179.43 Billion |
| Forecasted Market Value ( USD | $ 308.57 Billion |
| Compound Annual Growth Rate | 14.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |