Global Point-of-Care/Rapid Diagnostics Market - Key Trends and Drivers Summarized
Revolutionizing Healthcare: The Impact of Point-of-Care/Rapid Diagnostics
Point-of-care (POC) or rapid diagnostics are medical tests performed at or near the site of patient care, providing immediate results without the need for extensive laboratory infrastructure. These diagnostics cover a wide range of applications, including infectious disease detection, blood glucose monitoring, cardiac markers, and pregnancy testing. POC diagnostics typically involve simple procedures using portable devices or test kits that require minimal training to operate. For instance, lateral flow assays, commonly used in home pregnancy tests and COVID-19 antigen tests, allow for rapid visual results by detecting the presence of specific biomarkers in a sample. Other POC technologies, such as portable blood analyzers and handheld molecular testing devices, use advanced techniques like microfluidics and nucleic acid amplification to deliver precise and timely results. The core advantage of POC diagnostics is their ability to provide healthcare professionals with immediate, actionable information, facilitating faster clinical decisions and improving patient outcomes.How Are Point-of-Care/Rapid Diagnostics Utilized in Various Medical Settings?
Point-of-care diagnostics are integral to various medical settings, enhancing the efficiency and effectiveness of healthcare delivery. In emergency departments, POC tests are used to quickly diagnose conditions like myocardial infarction, enabling immediate intervention that can be critical in saving lives. In primary care and outpatient clinics, these diagnostics allow for on-the-spot testing and diagnosis of common conditions such as strep throat, influenza, and urinary tract infections, reducing the need for follow-up visits and streamlining patient care. Remote and rural healthcare facilities, often lacking advanced laboratory capabilities, rely heavily on POC diagnostics to provide essential medical services, bridging the gap in healthcare access. Additionally, POC diagnostics play a crucial role in chronic disease management, allowing patients to monitor conditions such as diabetes and coagulation disorders at home, thereby maintaining better control over their health. The versatility and rapid turnaround of POC diagnostics make them indispensable tools across diverse healthcare environments.What Are the Current Trends in Point-of-Care/Rapid Diagnostic Technology?
The field of point-of-care diagnostics is experiencing rapid advancements driven by technological innovations and the increasing demand for efficient healthcare solutions. One significant trend is the integration of digital health technologies, where POC devices are equipped with connectivity features to upload test results directly to electronic health records (EHRs) and mobile health applications, facilitating real-time data sharing and remote patient monitoring. The development of multiplex testing platforms is another trend, enabling the simultaneous detection of multiple biomarkers from a single sample, which is particularly useful for diagnosing co-infections or comprehensive health screenings. Advances in microfluidics and lab-on-a-chip technologies are leading to the creation of more compact and portable POC devices with enhanced sensitivity and specificity. Additionally, the use of artificial intelligence (AI) and machine learning algorithms is improving the accuracy and predictive capabilities of POC diagnostics, offering personalized insights based on patient data. These trends are revolutionizing the landscape of rapid diagnostics, making them more accessible, reliable, and integrated into the broader healthcare system.What Factors Are Driving the Growth in the Point-of-Care/Rapid Diagnostics Market?
The growth in the point-of-care/rapid diagnostics market is driven by several factors, reflecting the increasing need for timely and accurate diagnostic solutions. The rising prevalence of chronic diseases and infectious diseases, such as diabetes and COVID-19, is a significant driver, as POC diagnostics enable early detection and management of these conditions. Technological advancements are enhancing the functionality and user-friendliness of POC devices, encouraging their adoption across various healthcare settings. The growing emphasis on personalized medicine and the shift towards decentralized healthcare are also propelling market growth, as POC diagnostics support individualized treatment plans and facilitate care outside traditional clinical environments. Additionally, increasing investments in healthcare infrastructure and the expansion of telemedicine services are boosting demand for rapid diagnostic tools. Public health initiatives aimed at improving disease surveillance and healthcare accessibility in remote areas further support market expansion. These factors collectively ensure robust growth in the point-of-care/rapid diagnostics market, underscoring their critical role in modern healthcare delivery and disease management.Report Scope
The report analyzes the Point-of-Care/Rapid Diagnostics market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below.- Segments: Mode (OTC-based Testing, Prescription-based Testing); Platform (Lateral Flow Assays, Dipsticks, Microfluidics, Molecular Diagnostics, Immunoassays); Product (Glucose Monitoring Products, Cardiometabolic Testing Products, Infectious Disease Testing Products, Coagulation Testing Products, Pregnancy & Fertility Testing Products, Tumor/Cancer Marker Testing Products, Urinalysis Testing Products, Cholesterol Testing Products, Hematology Testing Products, Drugs-of-Abuse Testing Products, Fecal Occult Testing Products, Other POC Products).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Other POC Products segment, which is expected to reach US$3.3 Billion by 2030 with a CAGR of 6%. The Drugs-of-Abuse Testing Products segment is also set to grow at 6.3% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $8.1 Billion in 2024, and China, forecasted to grow at an impressive 11.1% CAGR to reach $10 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Point-of-Care/Rapid Diagnostics Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Point-of-Care/Rapid Diagnostics Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Point-of-Care/Rapid Diagnostics Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, AccuBioTech Co., Ltd., Becton, Dickinson and Company, Chembio Diagnostic Systems, Inc., Danaher Corporation and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 13 companies featured in this Point-of-Care/Rapid Diagnostics market report include:
- Abbott Laboratories
- AccuBioTech Co., Ltd.
- Becton, Dickinson and Company
- Chembio Diagnostic Systems, Inc.
- Danaher Corporation
- EKF Diagnostics Holdings PLC
- Instrumentation Laboratory
- Johnson & Johnson
- Nova Biomedical Corporation
- PTS Diagnostics
- Quidel Corporation
- Roche Diagnostics (Schweiz) AG
- Sekisui Diagnostics LLC
- Siemens AG
- Trinity Biotech PLC
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- AccuBioTech Co., Ltd.
- Becton, Dickinson and Company
- Chembio Diagnostic Systems, Inc.
- Danaher Corporation
- EKF Diagnostics Holdings PLC
- Instrumentation Laboratory
- Johnson & Johnson
- Nova Biomedical Corporation
- PTS Diagnostics
- Quidel Corporation
- Roche Diagnostics (Schweiz) AG
- Sekisui Diagnostics LLC
- Siemens AG
- Trinity Biotech PLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 376 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
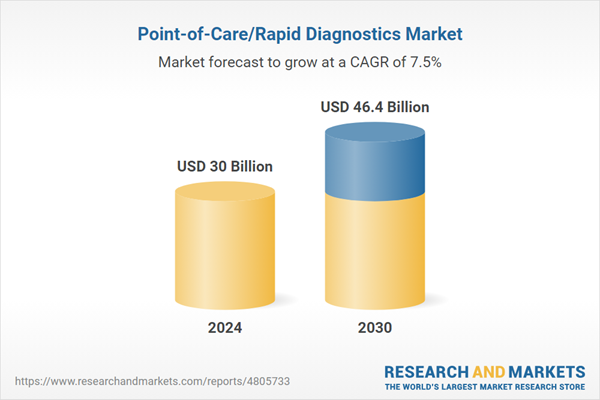
| Estimated Market Value ( USD | $ 30 Billion |
| Forecasted Market Value ( USD | $ 46.4 Billion |
| Compound Annual Growth Rate | 7.5% |
| Regions Covered | Global |