Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Hence, due to the surge in COVID-19 cases, the demand for respiratory devices increased, thereby fueling the growth of the market. However, as COVID-19 cases declined and the pandemic accelerated the use of respiratory devices, the studied market is now expected to project stable growth over the studied period. For instance, according to WHO, lung cancer is the leading cause of cancer deaths globally, with smoking responsible for 85% of cases. Early screening of high-risk individuals can improve survival rates, while primary prevention measures, such as tobacco control and reducing environmental risk factors, are crucial in lowering incidence and saving lives.
Key Market Drivers
Rising Prevalence of Respiratory Disorders
The escalating incidence of respiratory disorders worldwide is poised to propel the expansion of the Global Respiratory Endotherapy Devices Market. The rising prevalence of conditions such as chronic obstructive pulmonary disease (COPD), asthma, and respiratory infections is fostering an increased demand for advanced therapeutic interventions. Respiratory endotherapy devices play a pivotal role in the management of these disorders, offering minimally invasive and targeted treatments. For instance, According to the U.S. Centers for Disease Control and Prevention (CDC), smoking is responsible for approximately 8 out of 10 COPD-related deaths.Additionally, increasing levels of indoor and outdoor air pollution, along with exposure to dust and harmful chemicals, are significant contributors to the rising prevalence of COPD. As the global population ages and environmental factors contribute to respiratory health challenges, the need for effective and efficient endotherapy solutions is becoming more pronounced.
The market is witnessing a surge in technological innovations, including bronchoscopes, tracheoscopes, and other endoscopic devices, which enhance diagnostic accuracy and treatment precision. Healthcare providers are increasingly adopting these devices to address respiratory issues, contributing to the overall market growth. The growing awareness among patients and healthcare professionals regarding the benefits of endotherapy, such as reduced recovery times and improved patient outcomes, is driving the uptake of these devices. In conclusion, the escalating burden of respiratory disorders, coupled with technological advancements in endotherapy, is anticipated to foster significant growth in the Global Respiratory Endotherapy Devices Market in the coming years.
Key Market Challenges
High Cost of Respiratory Endotherapy Devices
The high cost associated with respiratory endotherapy devices is anticipated to pose a significant impediment to the growth of the Global Respiratory Endotherapy Devices Market. The substantial financial investment required for acquiring these sophisticated medical devices can act as a deterrent for healthcare institutions, particularly in regions with constrained healthcare budgets. The intricate technology and specialized materials involved in manufacturing respiratory endotherapy devices contribute to their elevated price point, limiting accessibility for certain healthcare facilities and hindering widespread adoption. The financial burden on patients seeking respiratory treatments may curb patient acceptance and usage.High device costs often translate to increased procedural expenses, potentially affecting reimbursement policies and posing challenges for both healthcare providers and patients. Market players need to address these cost-related concerns through strategic initiatives such as research and development aimed at cost reduction, exploring partnerships for shared investments, and advocating for favorable reimbursement policies. Mitigating the economic barriers associated with respiratory endotherapy devices is crucial for fostering market growth and ensuring broader access to these advanced medical solutions in the evolving landscape of respiratory healthcare.
Key Market Trends
Advancements in Minimally Invasive Technologies
The growth trajectory of the Global Respiratory Endotherapy Devices Market is poised to be significantly steered by continual advancements in minimally invasive technologies. As medical science evolves, there is a paradigm shift towards procedures that offer reduced patient trauma, shorter recovery times, and enhanced precision. Respiratory endotherapy devices are at the forefront of this transformative trend, providing cutting-edge solutions for the diagnosis and treatment of respiratory disorders with minimal invasiveness.Innovations in endoscopic technologies, including the development of high-definition imaging and advanced navigation systems, empower healthcare practitioners to conduct intricate respiratory procedures with unprecedented clarity and accuracy. The integration of robotics and artificial intelligence further augments the capabilities of respiratory endotherapy devices, enabling more precise interventions and personalized treatment strategies. This technological evolution not only enhances the efficacy of respiratory procedures but also contributes to increased patient acceptance. As the global healthcare landscape embraces these advancements, the demand for state-of-the-art respiratory endotherapy devices is expected to rise, driving market growth. In essence, the continual refinement of minimally invasive technologies is a pivotal catalyst propelling the expansion of the Global Respiratory Endotherapy Devices Market, shaping the future of respiratory healthcare.
Key Market Players
- Fujifilm Holdings Corporation.
- Medtronic plc.
- Johnson & Johnson.
- Smith & Nephew Plc.
- Stryker corporation.
- Olympus corporation.
- Hoya corporation.
- Steris Healthcare.
- Conmed corporation.
- Boston Scientific corporation.
Report Scope:
In this report, the Global Respiratory Endotherapy Devices Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Respiratory Endotherapy Devices Market, By Devices:
- Diagnostic Devices
- Therapeutic Devices
Respiratory Endotherapy Devices Market, By End User:
- Peripheral Vascular Intervention
- Endovascular Interventions
- Cardiac
- Interventional Radiology
- Venous
- Others
Respiratory Endotherapy Devices Market, By Specialist:
- Hospitals
- Diagnostic Centers
- Ambulatory Surgical Centers
Respiratory Endotherapy Devices Market, By Region:
- North America
- United States
- Canada
- Mexico
- Europe
- France
- United Kingdom
- Italy
- Germany
- Spain
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Respiratory Endotherapy Devices Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Fujifilm Holdings Corporation.
- Medtronic plc.
- Johnson & Johnson.
- Smith & Nephew Plc.
- Stryker corporation.
- Olympus corporation.
- Hoya corporation.
- Steris Healthcare.
- Conmed corporation.
- Boston Scientific corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | March 2025 |
| Forecast Period | 2024 - 2030 |
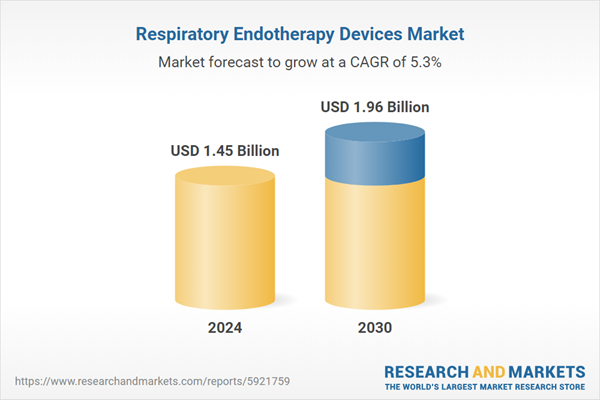
| Estimated Market Value ( USD | $ 1.45 Billion |
| Forecasted Market Value ( USD | $ 1.96 Billion |
| Compound Annual Growth Rate | 5.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |