The COVID-19 outbreak impacted the healthcare sector, as hospital and healthcare services were significantly reduced due to social distancing measures taken by the government in the country. Moreover, the COVID-19 pandemic affected the country's economy and adversely impacted the functioning of general hospital care for non-COVID-19 patients in hospitals. An article titled "The impact of COVID-19 on anesthesia and critical care services in the UK: a serial service evaluation", published in the Journal of Anaesthesia in September 2021, indicated that in January 2021, one-third of the anesthesia staff were unavailable, 42% of operating theaters were closed, and national surgical activity was reduced to less than half, including reduced cancer and emergency surgery. The outbreak of COVID-19 impacted the market, as hospitals and healthcare services were significantly reduced due to the social distancing measures enforced in the country. In addition, intensivists and anesthesiologists were teaming up to treat COVID-19 patients. As per an article published in June 2021, titled "Surgical activity in England and Wales during the COVID-19 pandemic: a nationwide observational cohort study", presented in the British Journal of Anaesthesia, the total number of surgical procedures carried out in England and Wales in 2020 was 3,102,674. Thus, such instances highlight that COVID-19 had a significant impact on the market's growth. However, the market is gaining traction owing to the resumption of elective surgeries and is likely to regain its pre-pandemic growth rate in the near future.
The growth in the geriatric population is likely to result in a significant increase in the demand for surgeries in the United Kingdom as this population is more vulnerable to severe diseases and, ultimately, surgeries and treatments. The overall increase in the number of surgeries performed has a strong impact on the anesthesia devices market in the United Kingdom, as anesthetic devices are used in surgical procedures to reduce pain.
The Health Foundation Report, updated in December 2021, showed that, in the next 25 years, the number of people aged over 85 would double to 2.6 million in the United Kingdom. Additionally, as per the Overview of the UK population: January 2021 data updated by the government of the United Kingdom, it is estimated that there will be an additional 7.5 million people aged 65 years and over in the United Kingdom in the next 50 years. Several age-related illnesses have been linked to the aging population, resulting in increased demand for surgeries coupled with generating demand for anesthesia devices in the operating room (OR) and intensive care unit (ICU), in turn driving the country's market.
Furthermore, the UK Factsheet January 2022, published by the British Heart Foundation, stated that around 7.6 million people were living with heart and circulatory diseases, and nearly 4 million males and 3.6 million females were living with heart and circulatory diseases. This highly prevalent heart and circulatory disease patients generate demand for surgical procedures to avoid fatalities among the population, further boosting the demand for anesthesia devices used during surgical procedures.
However, difficulties associated with the usage of anesthesia devices and issues regarding product recalls may restrain the market's growth over the forecast period.
UK Anesthesia Devices Market Trends
Anesthesia Monitors Sub-segment Expected to Register a High CAGR in the Forecast Period
Anesthesia monitors are used to record and display the delivery of anesthetic substances, like gases, drugs, and fluids, to the patient. During surgeries, these are used to check the patient's health and reaction to the indication of anesthesia. The integration of various functionalities, such as oxygen saturation level, carbon dioxide level, heart rate, and blood pressure, coupled with decision support systems and data analysis aids, allows clinicians to get better insights into their patients.In May 2021, the Association of Anaesthetists updated its guidelines for minimum standards for patient monitoring during anesthesia. The new guidelines emphasize the use of anesthesia monitors along with the monitoring of ECG, SpO2, NIBP, and capnography in the country. Such developments are expected to propel market segment growth over the coming years. Moreover, as per the British Association of Aesthetic Plastic Surgeons (BAAPS) report released in February 2021, approximately 15,000 cosmetic surgeries were carried out in the United Kingdom in 2021. Also, as per the source above, breast augmentation was the most popular cosmetic surgery among women in the country in 2020, with over 4.6 thousand treatments, followed by breast reduction with over 3.1 thousand procedures. In addition, with 413 operations performed in 2020, rhinoplasty was the most popular surgery among men in the same year. This is poised to drive the demand for anesthesia devices in the country, bolstering the market's growth.
Furthermore, the National Health Service (NHS) Key Statistics: England published in February 2022 reported that accidents and emergency (A&E) attendance had increased in England. As of January 2022, an average of 42,000 people went to major hospitals' A&E departments in England every day, and there were an average of 12,256 emergency admissions to hospitals via A&E each day. This increasing number of injuries and admissions in emergency settings for surgeries is expected to boost the growth of the studied market in the United Kingdom.
Similarly, initiatives by market players are another factor driving the market's growth. For instance, in January 2020, a ground-breaking regional anesthesia device, SAFIRA, developed by medical device company Medovate in collaboration with clinicians from the UK's National Health Service (NHS), was demonstrated live at Arab Health 2022. Thus, the abovementioned factors are expected to increase the market's growth.
Anesthesia Workstations Expected to Witness Healthy Growth in the Coming Years
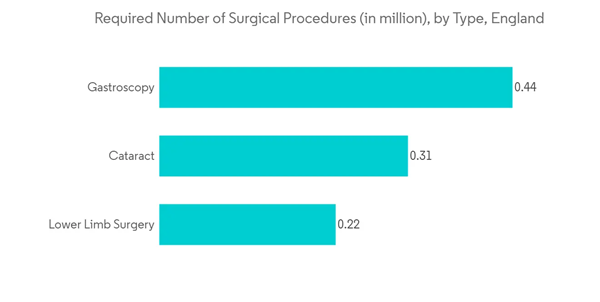
The traditional anesthesia machine has developed into a sophisticated care station throughout time. The new devices offer broad capabilities for breathing, monitoring, inhaled agent delivery, low-flow anesthesia, and closed-loop anesthesia by utilizing cutting-edge electronics, software, and technology.With the growing number of surgeries in the United Kingdom, the demand for anesthesia workstations is increasing. For instance, as per the survey results published in BioMed Central Journal in June 2022, it was observed that in England, 4.34 million elective surgeries were required as of March 2022; and 3.30 million (76.0%) of these patients were on a confidential waiting list. General surgery (1.52 million), orthopedics (0.97 million), and ophthalmology were the specialties with the most demand. Hence, with such a high number of surgical procedure requirements in England, there is expected to be a growth in demand for anesthesia workstations, fueling the segment growth.
Additionally, according to a study from the British Journal of Anaesthesia published in December 2021, in England and Wales, the total number of surgeries performed in 2021 was around 3.10 million, and about 1.56 million procedures were canceled. However, the number of surgical procedures performed reduced in 2020 due to the COVID-19 pandemic, and the backlog of these procedures is expected to boost the market over the forecast period.
UK Anesthesia Devices Industry Overview
The United Kingdom is one of the more developed regions in Europe, along with other developed nations like France and Germany. As a result, it enjoys the presence of most of the global players in the anesthesia devices market. Moreover, the country has a developed healthcare system, and the rate of adoption of newer technologies is higher. This makes this market more lucrative for innovation, which encourages companies to enter the UK market. Some of the prominent players in the market include B. Braun Melsungen AG, Medtronic PLC, Draegerwerk AG, Philips, and Fisher & Paykel Healthcare.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- B. Braun SE
- Medtronic PLC
- Draegerwerk AG
- Ambu AS
- Fisher & Paykel Healthcare
- GE Healthcare
- Koninklijke Philips NV
- Mindray Medical International Limited
- Smiths Medical
- Teleflex Inc.