The report United States Cardiovascular Devices Market & Forecast covers by Device Type (Diagnostic and Monitoring, Therapeutic and Surgical), Application (Coronary Artery Disease (CAD), Cardiac Arrhythmia, Heart Failure, Others), End-User (Hospitals and Clinics, Ambulatory Surgical Centers, Diagnostic Centers, Cardiac Catheterization Laboratories, Others), States and Company 2025-2033.
United States Cardiovascular Devices Market Outlook
Cardiovascular devices are medical instruments used to diagnose, monitor, and treat cardiovascular diseases (CVDs) that affect the heart and blood vessels. These devices include diagnostic tools like electrocardiograms (ECGs), echocardiograms, imaging systems, and therapeutic devices such as stents, pacemakers, defibrillators, and heart valves. Cardiovascular devices are essential in managing conditions such as coronary artery disease, arrhythmias, heart failure, and hypertension.Cardiovascular diseases are still a leading cause of death in the United States, and these devices are necessary for proper diagnosis and treatment. Stents open blocked arteries, pacemakers and defibrillators regulate heart rhythms in patients with arrhythmias, and advanced imaging systems allow doctors to assess heart function. Wearable devices and remote monitoring technologies enable continuous tracking of heart health. The growing aging population and increasing prevalence of lifestyle-related risk factors, such as obesity and smoking, drive the demand for cardiovascular devices. As innovations continue to evolve, the devices are improving patient outcomes and quality of life in the U.S.
Increasing Prevalence of Cardiovascular Diseases Growth Driver in the United States Cardiovascular Devices Market
A growing trend in cardiovascular diseases in the United States has become a significant growth driver for the cardiovascular devices market. Millions of Americans suffer from CVDs, such as coronary artery disease, heart failure, and arrhythmias, and therefore demand more diagnostic and therapeutic devices. Advanced cardiovascular devices such as stents, pacemakers, and defibrillators have remained a critical need in healthcare settings because of the population that is aging and lifestyle-related risk factors, such as obesity, hypertension, and diabetes. With this, more health care providers are resorting to these devices in a quest to enhance patient outcomes and to effectively manage chronic cardiovascular conditions. In 2022, 702,880 people died from heart disease. That's 1 in every 5 deaths.
Technological Innovation in Cardiovascular Devices
Technological advancements play an essential role in boosting the U.S. cardiovascular devices market. Technological advancement such as minimally invasive procedures, robotic-assisted surgeries, and wearable heart monitoring devices enhance the efficacy and acceptance of cardiovascular treatments. Newer-generation stents, extended battery life pacemakers, and AI-powered diagnostic tools can be used for highly specific, personalized care. All of these advancements improve the efficiency of diagnosis and treatment while reducing recovery times and further boost the adoption of cardiovascular devices in hospitals and clinics all over the U.S.Government Initiatives and Healthcare Funding
Government initiatives and increased funding for healthcare are the key growth drivers for the cardiovascular devices market in the United States. Funding through policies initiated under the Affordable Care Act and funding for heart disease research have opened up better access to advanced healthcare technologies. In addition, programs that focus on cardiovascular health improvement, such as the CDC's Million Hearts initiative, emphasize early detection, prevention, and treatment of CVDs. Reimbursement and insurance coverage for cardiovascular procedures are also becoming more accessible, which motivates hospitals and clinics to invest in new devices. All these factors have contributed to the continued growth and adoption of cardiovascular devices.Barriers in the United States Cardiovascular Devices Market
Expensive Advanced Cardiovascular Devices
One of the big challenges of this market in the United States is that some of these advanced cardiovascular devices are very expensive. Advanced technology often includes something like a minimally invasive stent, which is associated with very high prices, which can put robotic-assisted surgical tools, among other things, out of range for some healthcare facilities and certainly some patients, especially if they belong to smaller hospitals or have low budgets located in a more rural region. Furthermore, though insurance may cover up to some of the costs, patients pay out-of-pocket for significant portions, which may deter widespread applications of these devices.Government and Approval Issues
The timeline for FDA approval of these cardiovascular devices in the United States is lengthy and complicated. Many clinical trials and regulatory requirements imposed by the FDA have to be met by such manufacturers to ensure that such products are safe and will work effectively. This may lead to a delay in bringing new devices to the market. Additionally, the research, development, and regulatory compliance expenses are quite high, and thus add to the overall expense for the manufacturers. This will lead to a slowdown in innovation and decreased availability of the most advanced cardiovascular solutions to the healthcare providers.United States Cardiovascular Diagnostics And Monitoring Devices Market
Diagnostic and monitoring devices have dominated the United States cardiovascular devices market. This is due to their significant role in controlling and preventing sickness. The tools enable medical practitioners to diagnose cardiac conditions, monitor patients' health, and investigate the effectiveness of treatment. Given the rise in cardiovascular diseases, the need for diagnostic equipment is continually increasing. From ECG machines to portable heart monitors, they enable early detection and proactive control over cardiovascular conditions, thus contributing to their growth in the United States cardiovascular device market.United States Coronary Artery Disease Cardiovascular Devices Market
Coronary artery disease happens to be one of the most conventional applications for the United States cardiovascular devices market as it significantly influences public health. It thus drives a wide range of cardiac devices for diagnosis, management and even treatment of CAD. Including stents and angioplasty balloons for revascularisation to superior imaging technology coronary angio, the various machines align with the complex needs for most patients living with CAD, bringing awareness into further development of revolutionary cardiovascular technologies in the market.United States Diagnostic Centers Cardiovascular Devices Market
Diagnostic facilities are among the most used in the United States cardiovascular devices market because of their pivotal role in cardiovascular fitness evaluation and control. These facilities house diverse diagnostic devices, including echocardiography machines, stress tests, and cardiac catheterization labs, allowing for comprehensive assessment and accurate analysis of cardiac conditions. With early detection and specific evaluation essential in cardiovascular care, diagnostic centers are important hubs for patient assessment, propelling their full-size usage in the market.California Cardiovascular Devices Market
California is one of the distinguished players in the United States cardiovascular devices market. This is due to its robust healthcare surroundings and revolutionary panorama. Home to renowned research institutions, clinical facilities, and enterprise leaders, California fosters conducive surroundings for developing and commercializing modern cardiovascular technology. The state's lively biotechnology zone and strategic collaborations among academia and enterprise continue to contribute to ongoing improvements in cardiovascular care, solidifying California's recognition as a vital hub in the cardiovascular devices market.New York Cardiovascular Device Market
The New York cardiovascular device market is growing with the increasing prevalence of cardiovascular diseases (CVDs) and an aging population. With CVDs being the leading cause of death in the state, there is a growing demand for advanced diagnostic and treatment devices, such as stents, pacemakers, defibrillators, and imaging systems. Technological advancements like minimally invasive procedures and wearable heart monitoring devices drive market growth. Increased healthcare access, government initiatives, and robust healthcare infrastructure support adopting these devices. The New York market is expected to grow further with innovative cardiovascular solutions that improve patient care and outcomes.Washington Cardiovascular Device Market
The Washington cardiovascular device market is growing due to the increasing prevalence of CVDs and the development of medical technology. With CVDs accounting for the highest morbidity and mortality in the state, the demand for cardiovascular devices such as stents, pacemakers, defibrillators, and diagnostic imaging tools is growing. The adoption of minimally invasive procedures and wearable devices for heart monitoring is also driving market growth. Additionally, government health initiatives and a well-developed healthcare infrastructure support the use of these advanced cardiovascular solutions. The Washington market will continue to rise with time as innovative devices advance the outcomes of patients and the quality of care.Florida Cardiovascular Device Market
Florida's cardiovascular device market is also growing since CVDs have been recognized as one of the leading causes of death in the state. There is growing demand for the advanced cardiovascular devices, which include stents, pacemakers, defibrillators, and diagnostic imaging equipment, as the population ages. Technological innovations, including minimally invasive procedures and wearable heart monitoring devices, are driving market growth. Established healthcare infrastructure in Florida and initiatives taken by the government to improve the prevention and treatment of heart disease promote extensive adoption of these devices. Advances in cardiovascular care should further boost the market going forward.Major Players
The leading companies in the United States cardiovascular devices market are Abbott Laboratories, Boston Scientific Corporation, Edwards Lifesciences, Cardinal Health Inc., Medtronic PLC, GE Healthcare, Johnson & Johnson Services, Inc., and Siemens Healthcare GmbH.Device type - Market breakup in 2 viewpoints:
1. Diagnostic and Monitoring Devices2. Therapeutic and Surgical Devices
Application - Market breakup in 4 viewpoints:
1. Coronary Artery Disease (CAD)2. Cardiac Arrhythmia
3. Heart Failure
4. Others
End-User - Market breakup in 5 viewpoints:
1. Hospitals and Clinics2. Ambulatory Surgical Centers
3. Diagnostic Centers
4. Cardiac Catheterization Laboratories
5. Others
States - Market of 29 States Covered in the Report:
1. California2. Texas
3. New York
4. Florida
5. Illinois
6. Pennsylvania
7. Ohio
8. Georgia
9. New Jersey
10. Washington
11. North Carolina
12. Massachusetts
13. Virginia
14. Michigan
15. Maryland
16. Colorado
17. Tennessee
18. Indiana
19. Arizona
20. Minnesota
21. Wisconsin
22. Missouri
23. Connecticut
24. South Carolina
25. Oregon
26. Louisiana
27. Alabama
28. Kentucky
29. Rest of the United States
All the Key players have been covered from 3 Viewpoints:
- Overview
- Recent Development
- Revenue Analysis
Company Analysis:
1. Abbott Laboratories2. Boston Scientific Corporation
3. Edwards Lifesciences
4. Cardinal Health Inc.
5. Medtronic PLC
6. GE Healthcare
7. Johnson & Johnson Services, Inc.
8. Siemens Healthcare GmbH.
Table of Contents
Companies Mentioned
- Abbott Laboratories
- Boston Scientific Corporation
- Edwards Lifesciences
- Cardinal Health Inc.
- Medtronic PLC
- GE Healthcare
- Johnson & Johnson Services, Inc.
- Siemens Healthcare GmbH.
Methodology
In this report, for analyzing the future trends for the studied market during the forecast period, the publisher has incorporated rigorous statistical and econometric methods, further scrutinized by secondary, primary sources and by in-house experts, supported through their extensive data intelligence repository. The market is studied holistically from both demand and supply-side perspectives. This is carried out to analyze both end-user and producer behavior patterns, in the review period, which affects price, demand and consumption trends. As the study demands to analyze the long-term nature of the market, the identification of factors influencing the market is based on the fundamentality of the study market.
Through secondary and primary researches, which largely include interviews with industry participants, reliable statistics, and regional intelligence, are identified and are transformed to quantitative data through data extraction, and further applied for inferential purposes. The publisher's in-house industry experts play an instrumental role in designing analytic tools and models, tailored to the requirements of a particular industry segment. These analytical tools and models sanitize the data & statistics and enhance the accuracy of their recommendations and advice.
Primary Research
The primary purpose of this phase is to extract qualitative information regarding the market from the key industry leaders. The primary research efforts include reaching out to participants through mail, tele-conversations, referrals, professional networks, and face-to-face interactions. The publisher also established professional corporate relations with various companies that allow us greater flexibility for reaching out to industry participants and commentators for interviews and discussions, fulfilling the following functions:
- Validates and improves the data quality and strengthens research proceeds
- Further develop the analyst team’s market understanding and expertise
- Supplies authentic information about market size, share, growth, and forecast
The researcher's primary research interview and discussion panels are typically composed of the most experienced industry members. These participants include, however, are not limited to:
- Chief executives and VPs of leading corporations specific to the industry
- Product and sales managers or country heads; channel partners and top level distributors; banking, investment, and valuation experts
- Key opinion leaders (KOLs)
Secondary Research
The publisher refers to a broad array of industry sources for their secondary research, which typically includes, however, is not limited to:
- Company SEC filings, annual reports, company websites, broker & financial reports, and investor presentations for competitive scenario and shape of the industry
- Patent and regulatory databases for understanding of technical & legal developments
- Scientific and technical writings for product information and related preemptions
- Regional government and statistical databases for macro analysis
- Authentic new articles, webcasts, and other related releases for market evaluation
- Internal and external proprietary databases, key market indicators, and relevant press releases for market estimates and forecasts

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 125 |
| Published | February 2025 |
| Forecast Period | 2024 - 2033 |
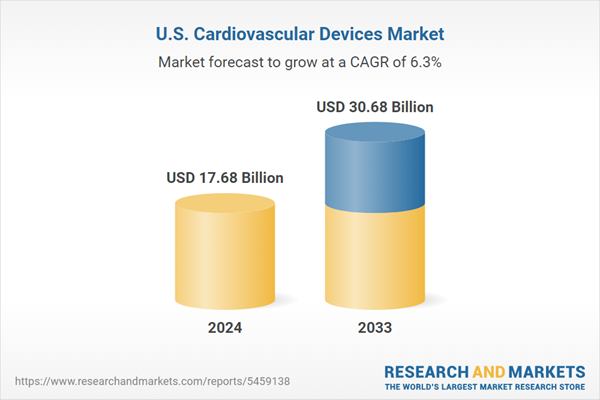
| Estimated Market Value ( USD | $ 17.68 Billion |
| Forecasted Market Value ( USD | $ 30.68 Billion |
| Compound Annual Growth Rate | 6.3% |
| Regions Covered | United States |
| No. of Companies Mentioned | 8 |