In a virtual paradigm, researchers can use digital tools to speed up hiring, cut down on overheads, and increase inclusivity. For patient-trial matching, for instance, cancer centers are starting to use artificial intelligence (AI). These platforms collect patient data from both structured and unstructured parts of electronic medical records, determine eligibility, and match patients to pertinent open studies in the ClinicalTrials.gov database. They use natural language processing and other AI approaches for this purpose.
The importance of these cutting-edge analytics tools in cancer is rising rapidly, as regulators work to enlarge previously stringent eligibility requirements. This factor is representing lucrative growth opportunities for the virtual clinical trials market in the United States. As a result of the COVID-19 pandemic, telemedicine has seen a sharp increase in usage in recent years. This is because most of the sponsors funding research studies have placed a greater emphasis on patient-centricity.
One of the biggest obstacles to patient enrollment and retention in clinical trials is the patient's geographic location and/or socioeconomic class. This is addressed via telemedicine, which also improves the possibility of better patient diversity. This provides a more realistic picture of a certain illness in a population and the potential effects of an investigational drug on that population. Furthermore, patients can interact with researchers as well as the treating doctors more effectively, when telemedicine is used in clinical research.
The pandemic switched research attention from study clinics to homes, leading researchers to consider novel methods for data gathering. The FDA published instructions to temporarily increase access to noninvasive medical devices for remote patient monitoring, such as electrocardiographs and spirometers. Researchers have changed the assessment frequency specifically in oncology. These developments are compatible with the virtual trial approach, which aims to increase the ability of studies to gauge patient-reported outcomes and produce real-world data. The U.S. market for virtual clinical trials has observed notable growth due to the pandemic.
U.S. Virtual Clinical Trials Market Report Highlights
- The interventional study design segment accounted for the highest market share in 2022 due to the rapid rise in the number of experiments being performed to develop novel medications for various diseases
- The expanded access segment is anticipated to experience maximum growth as it represents great potential
- The oncology segment dominated the market in 2022 with a revenue share of 57.73% owing to the rising cases of cancer in the United States
- The CNS indication segment is expected to witness maximum growth due to the increasing adoption of virtual clinical trials in this area
- The Phase II trials segment accounted for the highest revenue share in 2022 in the market due to many studies currently being under this study phase
- The Southern region dominated the U.S. market in 2022 with a revenue share of 33.25%, owing to a significant number of clinical trials being carried out in states such as Texas and Florida
- The Western region of the United States is anticipated to experience maximum growth during the forecast period, aided by the presence of states such as California that strongly focus on research activities
Table of Contents
Companies Mentioned
- ICON, plc
- Parexel International Corporation
- IQVIA
- Labcorp Drug Development
- Medidata Solutions
- Oracle
- Signant Health
- Medable, Inc.
- Halo Health Systems
- Science 37
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 100 |
| Published | June 2023 |
| Forecast Period | 2022 - 2030 |
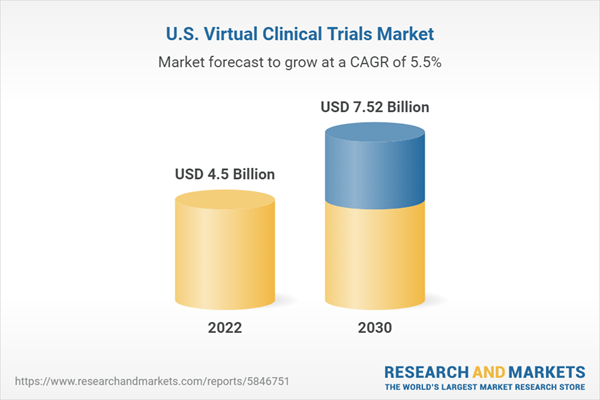
| Estimated Market Value ( USD | $ 4.5 Billion |
| Forecasted Market Value ( USD | $ 7.52 Billion |
| Compound Annual Growth Rate | 5.4% |
| Regions Covered | United States |
| No. of Companies Mentioned | 10 |