Therefore, the U.S. government spent around USD 239 billion every year from 2018 to 2019 on prevention and treatment of heart diseases. This includes the cost of medicines and health care services. Certain factors such as alcohol consumption, improper diet, and lack of exercise can cause cancer and heart-related diseases. Hence, the rapid increase in the incidence of cancer and cardiovascular disorders is likely to boost the demand for nuclear medicine in the U.S. The overall increase in the prevalence of cancer will likely boost the number of patients undergoing treatment, which increases the need for early diagnosis and treatment, thereby driving market growth.
Technological advancement in the nuclear medicine is a growth driver for the nuclear medicine market. Technetium-99m stands out as one of the most extensively utilized radioisotopes in the realm of radiopharmaceuticals. Alongside Technetium-99m, other widely employed radioisotopes include Iodine-131, Indium-111, and Fluorine-18. These medical isotopes play crucial roles in radiopharmacology, facilitating the study of drug distribution and movement within laboratory subjects. Furthermore, recent approvals by the FDA are anticipated to catalyze further expansion in the nuclear medicine market.
In September 2023, the U.S. FDA approved Technegas developed by Cyclopharm for use in nuclear medicine perfusion/ventilation (V/?) lung scans to diagnose pulmonary embolism. Technetium-99m (Tc-99m)-labeled Carbon Nanoparticles (CNP) are used in ventilation scintigraphy using the technology Technegas. Before undergoing lung imaging with a gamma camera, patients inhale the agent-produced nanoparticles, which are then aerosolized using on-site generators manufactured by Cyclopharm’s subsidiary Cyclomedica.
Rising demand for accurate diagnostic & treatment methods serves as a growth driver for the nuclear medicine market. Currently, available cancer treatment options, such as chemotherapy and radiation therapy, involve the use of drugs & radiation, which may not be completely accurate. Moreover, these therapies have a major disadvantage of affecting the whole body or the healthy organs surrounding the target site. Radioisotopes offer an advantage over these techniques as they can specifically target the tumor without affecting the surrounding body parts. Thus, benefits associated with these diagnostic and treatment procedures will likely boost the demand for radiopharmaceuticals in these fields. An increase in organizations undertaking initiatives to help address the growing need for nuclear medicine is anticipated to impel market growth. For instance, in August 2023, National Nuclear Security Administration (NNSA) was planning to spend tens of billions of dollars to modernize its nuclear-related research and production infrastructure over the next two decades in the U.S. This is expected to expand patient access to nuclear medicine procedures, which is estimated to improve their overall health and wellbeing.
Increasing prevalence of chronic diseases, such as cancer and cardiovascular diseases, and emergence of nuclear medicine as a better option to existing treatment methodologies for treatment of these diseases is anticipated to have a positive impact on market growth. According to National Center for Biotechnology Information (NCBI) in March 2023, around 20 million nuclear medicine & imaging procedures are conducted every year in the U.S. This rising volume of these procedures is expected to open new avenues in the market during the forecast period. To meet the increasing demand, companies are investing in R&D of new accelerator technology, detector systems, radiochemistry, imaging software, and automated processing modules for diagnosis and treatment of various diseases.
U.S. Nuclear Medicine Market Report Highlights
- Based on product, diagnostic products held the largest revenue share of 71.2% in 2023 owing to presence of large patient base and availability of advanced technologies such as Single-Photon Emission Computed Tomography (SPECT) and Positron Emission Tomography (PET)
- Based on application, the oncology segment dominated the market and is anticipated to maintain its dominance over the forecast period. This is attributed to the high prevalence of cancer and increased preference for radiopharmaceutical diagnosis. The probable approval of radiotherapeutic agents such as Iomab-B (indicated for AML), coupled with growing target patient population, is expected to drive market growth
- Based on end-use, the hospitals segment dominated the market and accounted for the largest revenue share. The segment is expected to continue its dominance over the forecast period. The nuclear medicine market in the U.S. is expanding due to the rise in the number of hospitals and clinics. The demand for nuclear medicine products is high owing to rising inpatient visits for diagnosis and treatment of diseases
Table of Contents
Companies Mentioned
- Eckert & Ziegler
- Curium
- GE Healthcare
- Jubilant Life Sciences Ltd.
- Bracco Imaging S.P.A
- Nordion (Canada), Inc.
- The Institute For Radioelements (IRE)
- NTP Radioisotopes SOC Ltd.
- Eczacıbaşı-Monrol
- Lantheus Medical Imaging, Inc.
- The Australian Nuclear Science and Technology Organization
- Novartis (Advanced Accelerator Applications)
- Siemens Healthineers AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 130 |
| Published | May 2024 |
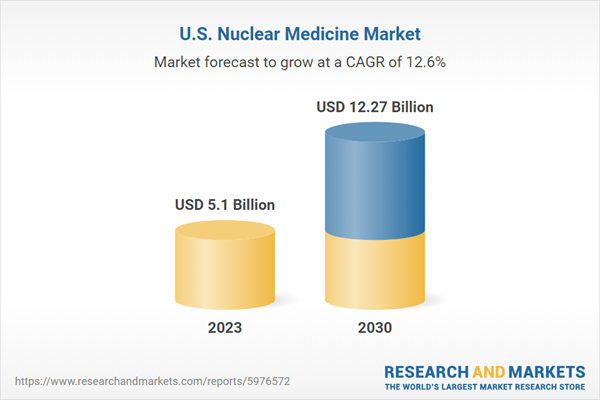
| Forecast Period | 2023 - 2030 |
| Estimated Market Value ( USD | $ 5.1 Billion |
| Forecasted Market Value ( USD | $ 12.27 Billion |
| Compound Annual Growth Rate | 12.6% |
| Regions Covered | United States |
| No. of Companies Mentioned | 13 |