The COVID-19 pandemic impacted the healthcare systems globally and significantly impacted the North American uterine cancer diagnostics and treatment market. For instance, according to the article published in November 2021 by Cancer Connect, doctors from Dana Farber Cancer Institute determined that during the COVID-19 pandemic, there was a 46.0% decrease in the diagnoses of the six most common cancer types - breast, colorectal, lung, pancreatic, gastric, and esophageal cancers in North America. Moreover, in the COVID-19 era, nearly 88.0% of the cancer care centers faced challenges in delivering usual cancer care for many reasons, including preventive measures, lack of personal protective equipment, and staff shortage, as per the NCBI research article published in 2021. However, with the resumption of all non-essential surgeries and medical requirements, the market has started to gain traction. It is expected to continue the upward trend over the forecast period.
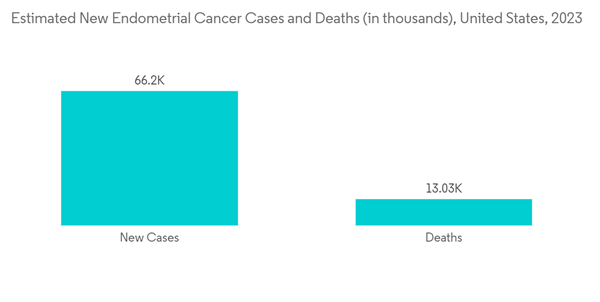
The major drivers for the market are the rising awareness about uterine cancer and the available therapies, increasing cases of uterine cancer, innovation in drug development, and subsequent technological advancements. For instance, according to the American Cancer Society estimates for cancer of the uterus in the United States for 2023, around 66,200 new cases of cancer of the body of the uterus (uterine body or corpus) will be diagnosed, and around 13,030 women will die from cancers of the uterine body in 2023. The same source also stated that endometrial cancer affects mainly post-menopausal women. The average age of women diagnosed with endometrial cancer is 60. It's uncommon in women under the age of 45. Thus, increasing cases of uterine cancer are expected to propel the demand for uterine cancer diagnostics and treatment, thereby propelling market growth.
The increasing product approval for treating uterine cancer diagnostics is also expected to propel the market growth over the forecast period. For instance, in April 2021, the U.S. FDA granted accelerated approval to Jemperli (dostarlimab) for treating patients with recurrent or advanced endometrial cancer that has progressed on or following prior treatment with platinum-containing chemotherapy and whose cancers have a specific genetic feature known as dMMR, as determined by an FDA-approved test. Similarly, Merck announced that the U.S. Food and Drug Administration (FDA) has approved KEYTRUDA, Merck’s anti-PD-1 therapy, as a single agent for the treatment of patients with advanced endometrial carcinoma that is microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR), as determined by an FDA-approved test, who have disease progression following prior systemic therapy in any setting and are not candidates for curative surgery or radiation. Thus, increasing product approvals is anticipated to positively impact the market growth over the forecast period.
However, the low success rate in clinical trials for cancer drugs, the high cost of research and development, and the high cost associated with the treatment will likely hinder the market growth over the forecast period.
North America Uterine Cancer Diagnostics & Treatment Market Trends
Immunotherapy Segment is Expected to Register Considerable Growth Over the Forecast Period
Immunotherapy is a treatment in which drugs help an individual’s immune system recognize and kill the cancer cells. Immunotherapy is done for treating certain forms of endometrial cancer that have spread or recurred. Immunotherapy is a treatment that uses a person's immune system to fight cancer. Immunotherapy can boost or change how the immune system works to find and attack cancer cells. Immunotherapy stimulates the endogenous immune response, specifically against tumor cells, and is the new frontier of anticancer treatment. Several compounds targeting different biological pathways are available. Some of these agents are already approved for treating non-gynecological malignancies like lung cancer and melanoma. They could also play a major role in the treatment of endometrial cancer.Increasing product approval and rising product launches are the major drivers for segment growth. For instance, in March 2022, Karyopharm Therapeutics Inc., a commercial-stage pharmaceutical company pioneering novel cancer therapies, provided an update on its discussions with the U.S. Food and Drug Administration (FDA) regarding its previously planned supplemental New Drug Application (sNDA) submission based on the data from the Phase 3 SIENDO study evaluating selinexor as a front-line maintenance therapy following chemotherapy in patients with advanced or recurrent endometrial cancer. Similarly, in March 2022, the Food and Drug Administration approved pembrolizumab (Keytruda, Merck) as a single agent for patients with advanced endometrial carcinoma that is microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR), as determined by an FDA-approved test, who have disease progression following prior systemic therapy in any setting and who are not candidates for curative surgery or radiation. Thus, owing to such instances, the segment is anticipated to have considerable segment growth over the forecast period.
United States is Expected to Witness Considerable Growth Over the Forecast Period
Key product launches, high concentration of market players or manufacturer's presence, acquisition & partnerships among major players, and increasing healthcare expenditure in the United States are some factors driving the growth of the United States uterine cancer diagnostics and treatment market. For instance, as per the United States Research and Development Funding and Performance: Fact Sheet, 2022, the R&D expenditures in the United States in 2021 were estimated at USD 580 billion, of which USD 96.5 billion was invested in basic research, USD 115.0 billion on applied research, and USD 368.5 billion was employed in the development sector. Such high investment is expected to accelerate the research, development, and approval of uterine cancer drugs in the country, propelling market growth.The increasing product approvals and launches in the country are also anticipated to increase market growth. For instance, in August 2022, Myovant Sciences and Pfizer received FDA approval in the United States for MYFEMBREE, a once-daily treatment for managing moderate to severe pain associated with endometriosis. MYFEMBREE is also approved for heavy menstrual bleeding associated with uterine fibroids in premenopausal women. Myovant and Pfizer will continue co-commercializing MYFEMBREE in the United States, and the product will be available immediately. Thus, owing to such factors, the country's market is expected to grow considerably.
North America Uterine Cancer Diagnostics & Treatment Industry Overview
The uterine cancer diagnostics and treatment market comprises global players that focus on the oncology division and are consolidated. Some major companies in this market include Merck & Co. Ltd, Pfizer Inc., and AbbVie Inc., among others. With the rising research by the pharmaceutical industry on the development of better therapeutics and diagnostics for various cancers, more companies are believed to enter the market in the coming future.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie Inc.
- Becton, Dickinson and Company
- Bristol-Myers Squibb Company
- F. Hoffmann-La Roche Ltd
- GlaxoSmithKline PLC
- Merck & Co. Inc.
- Novartis AG
- Pfizer Inc.
- Takeda Pharmaceutical Company Limited