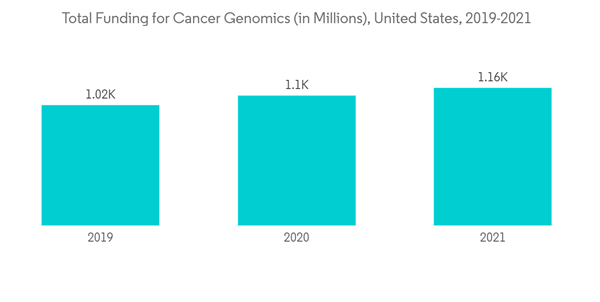Genome Editing Market is expected to grow with a healthy CAGR of nearly 14.4% during the forecast period (2019-2027).
The pandemic impacted the various clinical trial of the genome editing technology as the coronavirus pandemic is expected to put a strain on beds and other healthcare resources, no new patients are scheduled to start dosing in either study at this time. In May 2020 an article published titled "COVID-19 and gene editing: ethical and legal considerations" stated that gene editing could potentially be used on the genome of the virus that causes COVID-19, to make it harmless. It could be used to develop better testing kits, and could even be used to edit the human genome to prevent people from being infected by the virus. But gene editing is associated with a range of ethical issues such as safety, equal access, and consent. Bioethicists and researchers believe that gene editing in humans must be proven to be safe before it can be offered as a treatment option. There is also the issue of equal access to treatment, which must be considered. Hence, even though genome editing has advantages, the ethical concerns and the pandemic are affecting the market growth. Thus, the COVID-19 had impacted the growth by slightly slowing down the genome editing market pace over the forecast period.
Increasing prevalence of cancer and other genetic disorders, growing preference for personalized medicine, increase in private and public sector funding, rapid advancements in sequencing and Genome Editing technologies are some of the factors propelling the growth of the genome editing market. According to the National Center for Advancing Translational Sciences in 2019, the Somatic Cell Genome Editing (SCGE) Program at the National Institutes of Health (NIH) has awarded 24 more grants to researchers across the United States and Canada. The SCGE Program has awarded a total of USD 89 million in advance genome editing grants, over the next four years. This brings the total number of projects supported to 45, with approximately USD 190 million in funding spread out over six years. Such grants from the national institutes help to boost the market over the forecast period. Additionally, in May 2020, Rice University researchers have been awarded a four-year, USD 2.45 million grant from the National Institutes of Health (NIH) to support the development of a gene-editing treatment for sickle cell disease (SCD). The R01 grant, funded by the National Institutes of Health, aims to advance a method of modifying the stem cells responsible for producing damaged blood cells in SCD patients.
Moreover, in January 2020, Garner was allocated The National Institutes of Health (NIH) grant of more than USD 6 million to the fight against genetic diseases. Research studies are another factor in market growth. According to the article published in the Frontier in Genome Edition in March 2021, titled “CRISPR/Cas: Advances, Limitations, and Applications for Precision Cancer Research” CRISPR/Cas is a technology capable of making specific genome modifications in living eukaryotic cells. Sequence deletions, insertions, substitutions, integrations, and epigenetic gene regulation are all examples of genomic modifications.
Collaborations are another factor for the growth of the market. For instance, in November 2020, Eli Lilly partnered with Precision BioSciences to use ARCUS platform to research and develop potential in vivo therapies for genetic disorders, through a collaboration that could potentially generate about USD 2.7 billion for the Durham, NC, developer of therapies based on genome editing as well as “off-the-shelf” CAR T immunotherapies. Hence, such collaborations would increase market growth in the upcoming future.
However, the high cost of genomic equipment and ethical concerns related to genetic research will hinder the market growth.
The rise in funding and initiatives by the government to develop vaccines, medical technologies, drugs, and devices are further propelling the growth of the genome editing market globally. Research studies are another factor in market growth. According to the study titled “CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia” published in the New England Journal of Medicine in January 2021, CRISPR-Cas9-based gene editing is being tested to treat two cases of inherited diseases: one in a patient with TDT (β-Thalassemia) and the other in a patient with SCD (Sickle Cell Disease). During the 12-month period following the administration of CTX001, both patients experienced early, substantial, and sustained increases in fetal hemoglobin levels with more than 99% pancellularity. Thus, the application of CRISPR technology in Sickle Cell Disease and β-Thalassemia is expected to rise its demand over the forecast period.
Product launch is another factor in the market growth. In September 2020, The Tata CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) FELUDA test, powered by CSIR-IGIB (Institute of Genomics and Integrative Biology), FELUDA test, received regulatory approvals from the Drug Controller General of India (DCGI) for commercial launch, as per ICMR guidelines, test meeting high-quality benchmarks with 96% sensitivity and 98% specificity for detecting the novel coronavirus. This test uses an indigenously developed, cutting-edge CRISPR technology for the detection of the genomic sequence of the SARS-CoV-2 virus. CRISPR is a genome-editing technology for diagnosing diseases. Additionally, in December 2021, Thermo Fisher Scientific recently introduced its new protein, Invitrogen TrueCut HiFi Cas9 Protein, to complement its growing CRISPR gene editing solutions portfolio.
Thus, owing to the abovementioned factors are expected to drive the CRISPR technology market over the forecast period.
The Genome Editing market is dominated by North America due to the strong growth trend in the pharmaceuticals and biotechnology industries. Factors such as technological innovation in genome editing technology, increasing product approvals, and rising research and development procedures are expected to increase the market growth.
In March 2021, scientists at the University of California (UC), San Francisco, UC Berkeley, and UC Los Angeles have received the United States Food and Drug Administration approval to jointly launch an early phase, first-in-human clinical trial of a gene correction therapy in patients with sickle cell disease using the patient’s own blood-forming stem cells.
Similarly, in October 2020, Merck signed a licensing agreement with Takara Bio USA, Inc. Through this agreement Merck has licensed its CRISPR technology to Takara to develop vectors and other innovative products to support research using CRISPR as well cell engineering services, particularly stem cells.
Additionally, In March 2021, Health Canada proposed new guidelines for the Novel Food Regulations, specifically focused on plant breeding stating that gene-editing technology in agriculture is just as safe as conventional plant breeding. These guidelines provide more certainty for plant breeders and crop science companies. Such steps by the government are expected to expand the applications of CRISPR thus, boosting the studied market.
Additionally, in April 2022, a research study published titled "Transcription activator-like effector nuclease (TALEN) as a promising diagnostic approach for COVID-19" stated that TALEN has many advantages, such as unlimited target sites and high specificity. Compared to other methods, it has shown promising results and potential and serves as an essential genome editing tool for diagnostic work-up and treatment issues in infectious diseases. The characteristics defined for this technique are promising, i.e. potential for rapid, on-site, and non-expensive diagnostic work-up, especially during pandemics. Hence, such studies would increase the market growth in the region.
Thus, the abovementioned factors are likely to increase market growth in the upcoming future.
The market is partially fragmented and consists of several major players. Most of the players are based in developing counties due to more technological advancements. But due to the ease of connectivity in modern times, these players have also penetrated developing countries and are trying to establish a market in these countries as well. High growth potential in emerging regions provides new opportunities to industry players. Some of the companies which are currently dominating the market are GenScript USA Inc., Integrated DNA Technologies Inc., New England Biolabs Inc., Sangamo Biosciences Inc., and Thermo Fisher Scientific Inc. among others.
This product will be delivered within 2 business days.
The pandemic impacted the various clinical trial of the genome editing technology as the coronavirus pandemic is expected to put a strain on beds and other healthcare resources, no new patients are scheduled to start dosing in either study at this time. In May 2020 an article published titled "COVID-19 and gene editing: ethical and legal considerations" stated that gene editing could potentially be used on the genome of the virus that causes COVID-19, to make it harmless. It could be used to develop better testing kits, and could even be used to edit the human genome to prevent people from being infected by the virus. But gene editing is associated with a range of ethical issues such as safety, equal access, and consent. Bioethicists and researchers believe that gene editing in humans must be proven to be safe before it can be offered as a treatment option. There is also the issue of equal access to treatment, which must be considered. Hence, even though genome editing has advantages, the ethical concerns and the pandemic are affecting the market growth. Thus, the COVID-19 had impacted the growth by slightly slowing down the genome editing market pace over the forecast period.
Increasing prevalence of cancer and other genetic disorders, growing preference for personalized medicine, increase in private and public sector funding, rapid advancements in sequencing and Genome Editing technologies are some of the factors propelling the growth of the genome editing market. According to the National Center for Advancing Translational Sciences in 2019, the Somatic Cell Genome Editing (SCGE) Program at the National Institutes of Health (NIH) has awarded 24 more grants to researchers across the United States and Canada. The SCGE Program has awarded a total of USD 89 million in advance genome editing grants, over the next four years. This brings the total number of projects supported to 45, with approximately USD 190 million in funding spread out over six years. Such grants from the national institutes help to boost the market over the forecast period. Additionally, in May 2020, Rice University researchers have been awarded a four-year, USD 2.45 million grant from the National Institutes of Health (NIH) to support the development of a gene-editing treatment for sickle cell disease (SCD). The R01 grant, funded by the National Institutes of Health, aims to advance a method of modifying the stem cells responsible for producing damaged blood cells in SCD patients.
Moreover, in January 2020, Garner was allocated The National Institutes of Health (NIH) grant of more than USD 6 million to the fight against genetic diseases. Research studies are another factor in market growth. According to the article published in the Frontier in Genome Edition in March 2021, titled “CRISPR/Cas: Advances, Limitations, and Applications for Precision Cancer Research” CRISPR/Cas is a technology capable of making specific genome modifications in living eukaryotic cells. Sequence deletions, insertions, substitutions, integrations, and epigenetic gene regulation are all examples of genomic modifications.
Collaborations are another factor for the growth of the market. For instance, in November 2020, Eli Lilly partnered with Precision BioSciences to use ARCUS platform to research and develop potential in vivo therapies for genetic disorders, through a collaboration that could potentially generate about USD 2.7 billion for the Durham, NC, developer of therapies based on genome editing as well as “off-the-shelf” CAR T immunotherapies. Hence, such collaborations would increase market growth in the upcoming future.
However, the high cost of genomic equipment and ethical concerns related to genetic research will hinder the market growth.
Key Market Trends
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) is Expected to Hold a Significant Market Share Over the Forecast Period
The rise in funding and initiatives by the government to develop vaccines, medical technologies, drugs, and devices are further propelling the growth of the genome editing market globally. Research studies are another factor in market growth. According to the study titled “CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia” published in the New England Journal of Medicine in January 2021, CRISPR-Cas9-based gene editing is being tested to treat two cases of inherited diseases: one in a patient with TDT (β-Thalassemia) and the other in a patient with SCD (Sickle Cell Disease). During the 12-month period following the administration of CTX001, both patients experienced early, substantial, and sustained increases in fetal hemoglobin levels with more than 99% pancellularity. Thus, the application of CRISPR technology in Sickle Cell Disease and β-Thalassemia is expected to rise its demand over the forecast period.
Product launch is another factor in the market growth. In September 2020, The Tata CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) FELUDA test, powered by CSIR-IGIB (Institute of Genomics and Integrative Biology), FELUDA test, received regulatory approvals from the Drug Controller General of India (DCGI) for commercial launch, as per ICMR guidelines, test meeting high-quality benchmarks with 96% sensitivity and 98% specificity for detecting the novel coronavirus. This test uses an indigenously developed, cutting-edge CRISPR technology for the detection of the genomic sequence of the SARS-CoV-2 virus. CRISPR is a genome-editing technology for diagnosing diseases. Additionally, in December 2021, Thermo Fisher Scientific recently introduced its new protein, Invitrogen TrueCut HiFi Cas9 Protein, to complement its growing CRISPR gene editing solutions portfolio.
Thus, owing to the abovementioned factors are expected to drive the CRISPR technology market over the forecast period.
North America is Expected to Dominate the Market Over the Forecast Period
The Genome Editing market is dominated by North America due to the strong growth trend in the pharmaceuticals and biotechnology industries. Factors such as technological innovation in genome editing technology, increasing product approvals, and rising research and development procedures are expected to increase the market growth.
In March 2021, scientists at the University of California (UC), San Francisco, UC Berkeley, and UC Los Angeles have received the United States Food and Drug Administration approval to jointly launch an early phase, first-in-human clinical trial of a gene correction therapy in patients with sickle cell disease using the patient’s own blood-forming stem cells.
Similarly, in October 2020, Merck signed a licensing agreement with Takara Bio USA, Inc. Through this agreement Merck has licensed its CRISPR technology to Takara to develop vectors and other innovative products to support research using CRISPR as well cell engineering services, particularly stem cells.
Additionally, In March 2021, Health Canada proposed new guidelines for the Novel Food Regulations, specifically focused on plant breeding stating that gene-editing technology in agriculture is just as safe as conventional plant breeding. These guidelines provide more certainty for plant breeders and crop science companies. Such steps by the government are expected to expand the applications of CRISPR thus, boosting the studied market.
Additionally, in April 2022, a research study published titled "Transcription activator-like effector nuclease (TALEN) as a promising diagnostic approach for COVID-19" stated that TALEN has many advantages, such as unlimited target sites and high specificity. Compared to other methods, it has shown promising results and potential and serves as an essential genome editing tool for diagnostic work-up and treatment issues in infectious diseases. The characteristics defined for this technique are promising, i.e. potential for rapid, on-site, and non-expensive diagnostic work-up, especially during pandemics. Hence, such studies would increase the market growth in the region.
Thus, the abovementioned factors are likely to increase market growth in the upcoming future.
Competitive Landscape
The market is partially fragmented and consists of several major players. Most of the players are based in developing counties due to more technological advancements. But due to the ease of connectivity in modern times, these players have also penetrated developing countries and are trying to establish a market in these countries as well. High growth potential in emerging regions provides new opportunities to industry players. Some of the companies which are currently dominating the market are GenScript USA Inc., Integrated DNA Technologies Inc., New England Biolabs Inc., Sangamo Biosciences Inc., and Thermo Fisher Scientific Inc. among others.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
1 INTRODUCTION
4 MARKET DYNAMICS
5 MARKET SEGMENTATION (Market Size by Value - USD million)
6 COMPETITIVE LANDSCAPE
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Editas Medicine
- GenScript USA Inc.
- Horizon Discovery Group plc
- Integrated DNA Technologies Inc.
- Lonza Group Ltd
- Merck & Co.
- New England Biolabs Inc.
- Origene Technologies Inc.
- Sangamo Biosciences Inc.
- Takara Bio Inc.
- Thermo Fisher Scientific Inc.
- Transposagen Biopharmaceuticals Inc.
Methodology

LOADING...