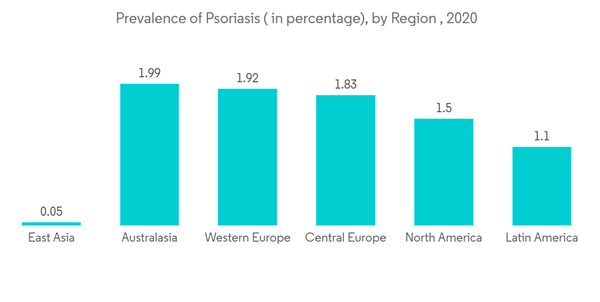
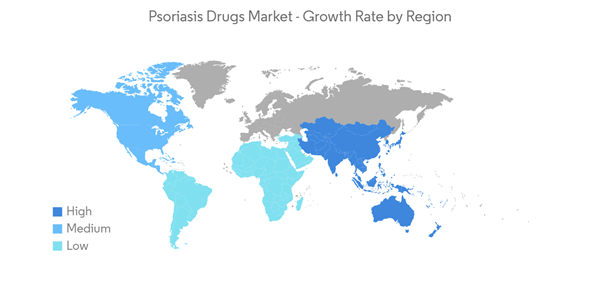
The COVID-19 pandemic initially had a negative growth impact on the psoriasis market, mainly because of the guidelines published by various regulatory bodies suggesting that patients receiving psoriasis treatment are at more risk of infecting COVID-19. In March 2020, the International Psoriasis Council (IPC) recommended physicians discontinue or postpone the prescription of immunosuppressant medications. However, later in September 2020, researchers from the Perelman School of Medicine at the University of Pennsylvania and 16 other research institutions from the United States and Canada, in collaboration with the National Psoriasis Foundation, created guidelines to care for patients with psoriasis during the coronavirus pandemic. The scientists found no evidence that medical interventions to treat psoriasis and psoriatic arthritis should be interrupted or altered to minimize COVID-19 risks. This guideline is further expected to resume the market growth.
An increasing disease burden and demand for psoriasis medicines in emerging economies and the increase in psoriasis research and pipeline drugs are the major factors responsible for the growth of the market. Novel drugs with oral administration open avenues for needle-averse patients. For instance, in November 2020, Bristol Myers Squibb Presents Late-Breaking Phase 2 Data Demonstrating the Safety and Efficacy of Deucravacitinib (BMS-986165) in Patients with Psoriatic Arthritis. This should be a driving factor for better uptake among patients averse to injectables.
Also, in February 2019, Janssen Biotech Inc., a biotechnology company, received the United States Food and Drug Administration approved TREMFYA for the treatment of severe plaque psoriasis. In addition, key players in the market are involved in various business strategies such as product launches to expand their product portfolios and gain a competitive edge in the market. For instance, in July 2020, Fujifilm Kyowa Kirin Biologics received the Japanese regulatory approval for manufacturing and marketing of the first adalimumab biosimilar in the island country for the indication of psoriasis vulgaris, arthritic psoriasis, pustular psoriasis, ankylosing spondylitis, entero-Behcet's disease, Crohn's disease, and polyarticular-course juvenile idiopathic arthritis. Owing to the above-mentioned factors, the market is expected to register a high growth rate during the forecast period. However, adverse side effects of most of the existing medication and the high cost of the treatment are the factors expected to hamper the market growth.
Key Market Trends
Interleukin Inhibitors are Expected to Register a High CAGR During the Forecast Period
Interleukin inhibitors are anticipated to witness the fastest growth during the forecast period. The factors aiding interleukin inhibitors are their improved safety and efficacy when compared to other classes of psoriasis drugs, which is consistently leading to increased adoption among patients. For instance, as per Eli-Lilly annual report 2020, Taltz generated worldwide revenue of USD 1.788 billion , an increase of 31% compared with the full year 2019. U.S. revenue was USD1.289 billion , an increase of 27%. Major approved antibodies targeting Interlunkins include Taltz (Ixekizumab), Cosentyx (Secukinumab), Siliq (Brodalumab), Tremfya (Guselkumab), Ilumya (Tildrakizumab), and SKYRIZI (Risankizumab).
In March 2019, AbbVie Inc., one of the key players in the market, received approval from the Japanese Ministry of Health Labour and Welfare (MHLW) for SKYRIZI (Risankizumab), an interleukin-23 (IL-23) inhibitor for treating plaque psoriasis, generalized pustular psoriasis, and erythrodermic psoriasis, among others. All these approvals in the market are further propelling the growth of the segment.
However, as psoriasis is a non-emergency condition, the ongoing COVID-19 pandemic has impacted the market as most of the interleukin R&D activities have been halted to focus on COVID-19 vaccine development.
North America Dominates the Market and is Expected to do so in the Forecast Period
North America has been one of the worst affected regions by the ongoing COVID-19 pandemic. The growth of the psoriasis market is expected to slow down to some extent because patients are avoiding visiting dermatology clinics for psoriasis treatment. Apart from restrictions on non-emergency medical services, new rules of the governments across this region have forced several players in the dermatology drug market to pause their business operations, which has impacted the growth of the market.
North America is expected to dominate the psoriasis market during the forecast period. In North America, the United States holds the largest market share owing to the high prevalence of psoriasis. As per the article, Prevalence of Psoriasis in Children and Adolescents in the United States: according to the article published in the JAMA Network 2021, an estimated 7.55 million US adults were living with psoriasis. Psoriasis prevalence was similar between women and men, with 3.2% in women and 2.8% in men. Other factors that will propel the market include the presence of favorable government initiatives, the presence of developed healthcare infrastructure, etc.
Owing to the increasing prevalence of targeted disease and due to the wide acceptance in North American countries, MC2 Therapeutics, a Europe-based company, in August 2020, signed a collaboration agreement with EPI Health LLC for commercialization of its WYNZORA Cream (calcipotriene and betamethasone dipropionate, w/w 0.005%/0.064%) in the US market. The WYNZORA Cream was approved in July 2020 by the United States Food and Drug Administration (FDA) for the topical indication of plaque psoriasis in adults. The upcoming launch of WYNZORA Cream in the American market may further propel the market growth in this region.
Competitive Landscape
The psoriasis drugs market is moderately competitive and consists of several key players. With respect to the market share, few of the key players currently dominate the market. Companies are focusing on business expansion in developing regions or emerging markets, such as India, China, and South Korea, by adopting strategies, including alliances and acquisitions, for the development of novel products. For instance, in August 2019, Amgen acquired Otezla, a biopharmaceutical company involved in the production of psoriasis drugs, for a value of USD 13.40 billion. Also, in July 2019, Cipla entered a partnership with Alvotech, a biopharmaceutical company, to commercialize AVT02. AVT02 is an adalimumab biosimilar for plaque psoriasis and rheumatoid arthritis.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
1 INTRODUCTION1.1 Study Assumptions and Market Definition
1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
4.1 Market Overview
4.2 Market Drivers
4.2.1 Increasing Disease Burden and Demand for Psoriasis Medicines in Emerging Economies
4.2.2 Increasing Use of Combination Therapies
4.2.3 Increase in Psoriasis Research and Pipeline Drugs
4.3 Market Restraints
4.3.1 Adverse Side Effects of Existing Medications
4.3.2 High Cost of Psoriasis Treatments
4.3.3 Extensive Drug Development and Approval Process
4.4 Porter's Five Forces Analysis
4.4.1 Threat of New Entrants
4.4.2 Bargaining Power of Buyers/Consumers
4.4.3 Bargaining Power of Suppliers
4.4.4 Threat of Substitute Products
4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD million)
5.1 By Type of Treatment
5.1.1 Biologic Drugs
5.1.2 Small Molecule Systemic Drugs
5.1.3 Tropical Therapies
5.2 By Mechanism of Action
5.2.1 TNF Alpha Inhibitors
5.2.1.1 Etanercept
5.2.1.2 Certolizumab Pegol
5.2.1.3 Adalimumab
5.2.1.4 Infiximab
5.2.1.5 Golimumab
5.2.2 PDE4 Inhibitors
5.2.2.1 Apremilast
5.2.3 Interleukin Inhibitors
5.2.3.1 Secukinumab
5.2.3.2 Ustekinumab
5.2.3.3 Other Interleukin Inhibitors
5.2.4 Other Mechanisms of Action
5.3 By Route of Administration
5.3.1 Oral
5.3.2 Parenteral
5.3.3 Topical
5.4 Geography
5.4.1 North America
5.4.1.1 United States
5.4.1.2 Canada
5.4.1.3 Mexico
5.4.2 Europe
5.4.2.1 Germany
5.4.2.2 United Kingdom
5.4.2.3 France
5.4.2.4 Italy
5.4.2.5 Spain
5.4.2.6 Rest of Europe
5.4.3 Asia-Pacific
5.4.3.1 China
5.4.3.2 Japan
5.4.3.3 India
5.4.3.4 Australia
5.4.3.5 South Korea
5.4.3.6 Rest of Asia-Pacific
5.4.4 Middle-East and Africa
5.4.4.1 GCC
5.4.4.2 South Africa
5.4.4.3 Rest of Middle-East and Africa
5.4.5 South America
5.4.5.1 Brazil
5.4.5.2 Argentina
5.4.5.3 Rest of South America
6 COMPETITIVE LANDSCAPE
6.1 Company Profiles
6.1.1 AbbVie Inc.
6.1.2 Amgen Inc.
6.1.3 AstraZenca
6.1.4 Biogen Idec
6.1.5 Boehringer Ingelheim
6.1.6 Celgene Corporation
6.1.7 Dr.Reddy`s Laboratories
6.1.8 Eli Lilly and Company
6.1.9 Forward Pharma
6.1.10 Johnson and Johnson (Janssen Biotech Inc.)
6.1.11 Leo Pharma AS
6.1.12 Merck and Co. Inc.
6.1.13 Novartis AG
6.1.14 Pfizer Inc.
6.1.15 Stiefel Laboratories Inc.
6.1.16 Sunpharmaceutical Industries Limited
6.1.17 Takeda Pharmaceutical Company Limited
6.1.18 UCB SA
6.1.19 Valeant Pharmaceuticals
7 MARKET OPPORTUNITIES AND FUTURE TRENDS
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie Inc.
- Amgen Inc.
- AstraZenca
- Biogen Idec
- Boehringer Ingelheim
- Celgene Corporation
- Dr.Reddy`s Laboratories
- Eli Lilly and Company
- Forward Pharma
- Johnson and Johnson (Janssen Biotech Inc.)
- Leo Pharma AS
- Merck and Co. Inc.
- Novartis AG
- Pfizer Inc.
- Stiefel Laboratories Inc.
- Sunpharmaceutical Industries Limited
- Takeda Pharmaceutical Company Limited
- UCB SA
- Valeant Pharmaceuticals