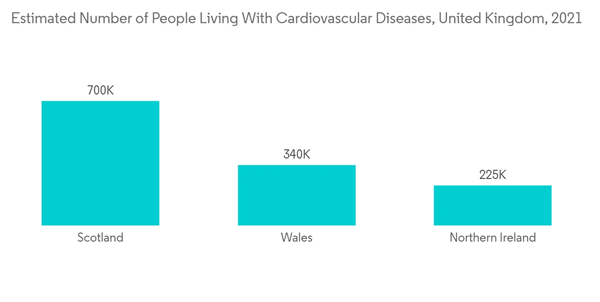
Like many other markets, the market was impacted by the COVID-19 pandemic. Various cardiovascular procedures and non-essential treatments were postponed. In March 2022, a research study was published in the Annals of Thoracic Surgery, which analyzed 717,103 adult cardiac surgery patients and more than 20 million COVID-19 patients stated that there was a 52.7% reduction in adult cardiac surgery volume and a 65.5% reduction in elective cases. The Mid-Atlantic region was most affected by the first COVID-19 surge, with a 69.7% reduction in overall case volume and an 80.0% reduction in elective cases. In the Mid-Atlantic and New England regions, the observed-to-expected mortality for isolated coronary bypass increased as much as 1.48 times (148% increase) pre-COVID rates. After the first COVID-19 surge, nationwide cardiac surgical case volumes did not return to baseline, indicating a COVID-19-associated deficit in cardiac surgery patients. Atherectomy is a procedure used to clear out clogged heart arteries. The cardiac surgeries would also include atherectomy surgeries. Hence, the market was affected across the globe. Hence, with the easing of COVID-19 restrictions, worldwide, the market gained traction and is expected to bounce back to its full potential over the years.
The market for atherectomy devices is majorly driven by the increasing preference for minimally invasive surgeries. Research studies about the expansion of minimally invasive surgeries are another factor that drives the market growth. In October 2021, a research study was published in Elsevier Journal, stating that continuous progress in percutaneous coronary intervention (PCI) has inspired surgeons to reduce the invasiveness of surgical revascularization techniques, resulting in the development of minimally invasive cardiac surgery (MICS) procedures, which have widely expanded over the past couple of decades. The main goals of MICS are to avert any form of sternotomy, reduce postoperative blood product transfusion, shorten ventilation times, reduce intensive care and hospital stays, diminish postoperative pain, and accelerate the return to normal activities. Hence, due to such advantages of MICS, there will be growth in the adoption of such surgeries which would increase the market growth.
Additionally, continuous product development and commercialization are expected to propel the growth of the market. In November 2021, Royal Philips launched peripheral and coronary artery applications, including the Nexcimerlaser system in Orlando, United States. The new Philips Laser System - Nexcimer- offers plug-and-play simplicity for coronary and peripheral atherectomy and lead extraction procedures. It is the only system compatible with catheters with Level I clinical data for ISR atherectomy and that can also support lead extraction procedures (the removal of pacemaker or defibrillator leads around the heart).
In the current trend of healthcare, reimbursement, and coverage reform, payer and provider consolidation for escalating healthcare costs are essential for patients and their caregivers. Reimbursement and coverage reform enhances the chances of opting for the treatment if it is covered. Hence, increasing reimbursement would increase the market growth in the upcoming period. In 2022, the Centers for Medicare and Medicaid Services (CMS), has increased reimbursement for peripheral intravascular lithotripsy (IVL) procedures. Procedure including revascularization, endovascular, open or percutaneous, lower extremity arteries, except tibial/peroneal; with intravascular lithotripsy, includes angioplasty within the same vessel (s) (CPT code: C9764) will receive USD 10,258. Similarly, a procedure including revascularization, endovascular, open or percutaneous, lower extremity arteries, except tibial/peroneal; with intravascular lithotripsy, and transluminal stent placement(s), includes angioplasty within the same vessels, (CPT code: C9765) will receive USD 16,402. Furthermore, processes including revascularization, endovascular, open or percutaneous, lower extremity arteries), except tibial/peroneal; with intravascular lithotripsy and transluminal stent placement(s), and atherectomy, including angioplasty within the same vessels, when performed (CPT code: C9767) will receive USD 16,402. Thus, all the above-mentioned factors are expected to boost the market over the forecast period.
However, the stringent regulatory scenarios may restrain the market growth over the forecast period.
Atherectomy Devices Market Trends
Directional Atherectomy Segment Holds a Significant Market Share in the Studied Market Over the Forecast Period
The directional atherectomy segment of the market is expected to witness significant growth in the forecast period over other products as it offers certain advantages such as low treatment cost, improved efficiency, etc. The increasing adoption of minimally invasive atherectomy procedures may boost the demand for atherectomy devices. According to a research study published by Krishna J. Rocha-Singh et. al., in June 2021, plaque removal with directional atherectomy (DA) before drug-coated balloon (DCB) angioplasty is a safe and effective treatment technique with a low provisional stent rate in patients with symptomatic severely calcified femoropopliteal artery disease. Thus, research studies like these are further expected to promote the usage of DA systems which is anticipated to fuel growth in the studied segment.The strategic initiatives taken by key market players such as product launches, mergers and acquisitions, and partnerships are also expected to contribute to market growth. For instance, in August 2021, Medtronic submitted an application to the USFDA for the approval of the TurboHawk Plus Directional Atherectomy System, an updated version of its older and largely phased-out atherectomy device. This device will facilitate increased atherectomy procedures during Peripheral Arterial Disease (PAD) interventions. Additionally, in November 2021, Avinger Inc. received 510(k) approval from the United States FDA for the Pantherisimage-guided atherectomy system's new clinical indication. This clearance allows the company to directly market Pantheris forthe treatment of in-stent restenosis (ISR) in the lower extremity arteries.
Therefore, overall, with the growing number of surgeries, and product innovation, the directional atherectomy systems segment is expected to grow during the forecast period of the study.
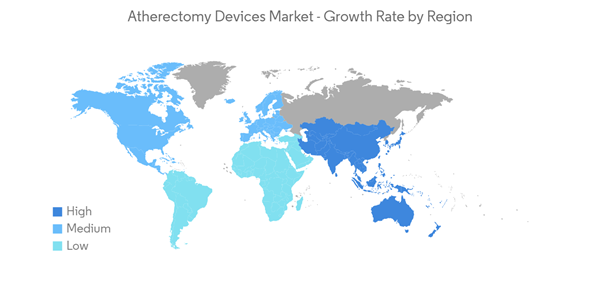
North America Holds a Significant Share in the Market and Expected to do Same during the Forecast Period
North America is expected to hold a significant share of the market. Factors such as increasing availability of reimbursements for atherectomy systems, adoption of atherectomy systems among medical professionals, the large patient population of peripheral and coronary artery diseases, and an increasing number of clinical trials (that aim to evaluate the therapeutic efficacy of atherectomy systems in specific disease treatment) are driving the growth of the North American atherectomy devices market.According to the February 2022 update of the CDC, in the United States, heart diseases are the leading cause of death irrespective of gender, race, or ethnicity and the most common type of heart disease is coronary heart disease (CHD) or coronary artery disease in which coronary arteries become too narrow or cholesterol blockages develop in the walls, leading to stroke or heart attack. Thus, due to the rising prevalence of heart diseases, the demand for atherectomy procedures is expected to increase in the region which is anticipated to fuel growth in the studied market over the forecast period.
A suitable reimbursement scenario and a high number of FDA-approved devices are the key reasons for this dominance. For instance, in October 2021, BD (Becton, Dickinson and Company) announced it has received 510(k) clearance for expanded indications from the U.S. FDA for the Rotarex Atherectomy System. Additionally, in November 2021, Royal Philips announced the North American debut of new peripheral and coronary artery applications, including the IntraSight Mobile system and the Nexcimer laser system.
Thus, all above mentioned factors expected to boost the market in the region over the forecast period.
Atherectomy Devices Market Competitor Analysis
The atherectomy devices market is moderately competitive, with the presence of many global players. For instance, Abbott Laboratories, B. Braun SE, Boston Scientific, CR Bard, Cardinal Health Inc., Koninklijke Philips NV, Medtronic PLC, Terumo Corporation, etc., are offering their products across the globe.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- B. Braun SE
- Boston Scientific Corporation
- Becton, Dickinson and Company
- Cardinal Health Inc.
- Koninklijke Philips NV
- Medtronic
- RA Medical Systems
- Terumo Corporation
- Cardiovascular Systems Inc.
- Biomerics LLC
- AngioDynamics Inc.