The COVID-19 pandemic significantly impacted the growth of the medical aesthetic devices market in early 2020 due to the lockdown imposed and many aesthetic procedures being canceled. An article published in the Journal of Clinics in Dermatology in June 2021 indicated that various procedures, such as deep peeling and laser treatments, were avoided during the COVID-19 pandemic because of the disruptions in the skin barrier. Also, the procedures, such as high-intensity focused ultrasound, fractional radiofrequency, and cryolipolysis equipped with stainless steel, gold, or plastic handpieces and probes, were postponed due to the risk of contamination.
However, aesthetic devices that consumers could use in their homes increased after the COVID-19 pandemic. According to an article published in the International Journal of Trichology in February 2022, home-based aesthetic devices have increased in recent years. They became more relevant during the COVID-19 pandemic, as more patients were hesitant to visit clinics for non-emergency reasons.
Major factors such as the increasing obese population, awareness regarding the aesthetic procedure, rising adoption of minimally invasive devices, and technological advancement in aesthetic devices are propelling the growth of the market.
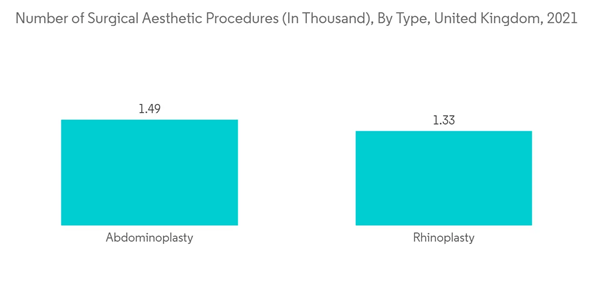
The increasing volume of aesthetic procedures worldwide is also contributing to the market's growth. For instance, according to the ISAPS survey released in December 2021, 10,129,528 surgical procedures and 14,400,347 nonsurgical cosmetic procedures were performed around the globe in 2020. Furthermore, according to the BAAPS survey released in February 2021, 15,405 cosmetic surgical procedures were performed in the United Kingdom in 2021. The increasing number of aesthetic procedures requires medical aesthetic devices. This is creating the need for medical aesthetic devices and driving the market's growth.
Also, the growing adoption of minimally invasive and non-invasive aesthetic procedures drives the market studied. As per an article published in the Journal Archives of Plastic Surgery in November 2021, the demand for aesthetic procedures was on the rise all over the world, especially in East Asian countries. With the advent of the internet, information became accessible to everyone, and people started becoming more aware of aesthetic procedures. All these factors together increased the public's awareness of medical aesthetic procedures, which, in turn, increased the sales of medical aesthetic devices. This is resulting in a huge growth in the market size of medical aesthetic devices.
Additionally, the approval of products by various regulatory authorities is also boosting the market's growth. For instance, in January 2022, Lumenis launched the Splendor X device, CE cleared for hair removal, vascular treatments, pigmented lesions, and wrinkles in the United Kingdom.
Thus, owing to the abovementioned factors, the market studied is expected to grow during the forecast period. However, social stigma and poor reimbursement scenarios may hinder the market's growth.
Medical Aesthetics Market Trends
Botulinum Toxin is Expected to Witness the Significant Growth During the Forecast Period
Botulinum toxin is a neurotoxic protein produced by the bacterium clostridium botulinum. The botulinum toxin injections tend to block the nerve signals to the muscle in which it is injected. As the muscle cannot contract without a signal, it diminishes or decreases unwanted facial wrinkles or appearance. As a result, highly diluted concentrations of botulinum toxin are used for cosmetic and non-cosmetic purposes, such as for treating frown lines between the eyebrows, dystonia, chronic migraine, and other purposes.Factors like the popularity of botulinum toxin procedures among young adults, the rise in demand for minimally invasive cosmetic methods, a higher number of beauty-conscious populations, and the launch of products are propelling the growth of the market segment.
The American Society of Plastic Surgeons survey was released in June 2022 in the United States. It reported that botulinum toxin Type A is one of the top minimally invasive cosmetic procedures among patients during 2021-2022. It also reported that patients aged 31- 45 year most commonly seek botulinum toxin type A cosmetic procedures. Additionally, an ISAPS survey released in December 2021 revealed that among all the non-surgical cosmetic procedures performed globally in 2020, 43.2% were Botulinum Toxin procedures. It also reported that a total of 6,213,859 botulinum toxin procedures were performed. Such a high adoption of botulinum toxin procedures creates more demand for it and thus drives the growth of the market segment.
The major players in the market are also investing more in the import and export of botulinum toxin products and are keenly investing in technological advancements. For example, in September 2022, Revance Therapeutics Inc. received approval from USFDA approved DAXXIFY (DaxibotulinumtoxinA-lanm) for injection for the temporary improvement of moderate to severe frown lines (glabellar lines) in adults.
Thus, owing to the abovementioned factors, the botulinum toxin segments are expected to project significant growth during the forecast period.
North America Holds Significant Share and Expected to do the Same During the Forecast Period
North America's medical aesthetic devices market is expected to project significant growth owing to the increasing volume of different aesthetic procedures, rising awareness about aesthetic and minimally invasive procedures, and technological advancement in the region.The large volume of aesthetic procedures in the region creates the need for aesthetic devices and thus drives the growth of the market. According to the AAFPRS survey released in March 2022, an estimated 1.4 million surgical and non-surgical procedures were performed in the United States in 2021, an increase of 40% over the procedures performed in 2020. Additionally, according to the ISAPS survey released in December 2021, 860,718 aesthetic procedures were performed in Mexico in 2021, of which 456,489 were aesthetic surgical procedures and 404,229 were non-surgical aesthetic procedures. Thus, the increasing number of cosmetic procedures among the population is anticipated to boost the growth of the market during the forecast period.
Also, the high concentration of key players in the region and subsequent product launches play a vital role in the growth of the market in the region. For instance, in April 2021, Alma Lasers launched Alma PrimeX, the ultimate non-invasive platform for body contouring and skin tightening.
Additionally, strategic initiatives are taken by the market players to strengthen their position also boosts the growth of the market in the region. For instance, in November 2021, NanoPass Technologies and Aesthetic Management Partners (AMP) signed an agreement to commercialize NanoPass's MicronJet 600 intradermal delivery device in the United States.
Thus, the aforementioned factors are expected to boost the market during the forecast period in North America.
Medical Aesthetics Industry Overview
The medical aesthetic devices market is moderately competitive and consists of many players. Companies like Abbvie Inc. (Allergan PLC), Alma Lasers Ltd (Sisram Med), Bausch Health Companies Inc. (Solta Medical Inc.), Cutera Inc., El. En. (Asclepion Laser Technologies), Lumenis Inc., Sciton Inc., and Syneron Medical Ltd hold a substantial share in the market.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbvie Inc. (Allergan PLC)
- Alma Lasers (Sisram Med)
- Bausch Health Companies Inc. (Solta Medical Inc.)
- Cutera
- El.En. S.p.A. (Asclepion Laser Technologies)
- Cynosure
- Boston Scientific Corporation (Lumenis Inc.)
- Sciton Inc.
- Candela Corporation
- Venus Concept
- Johnson & Johnson Private Limited
- Merz Pharma