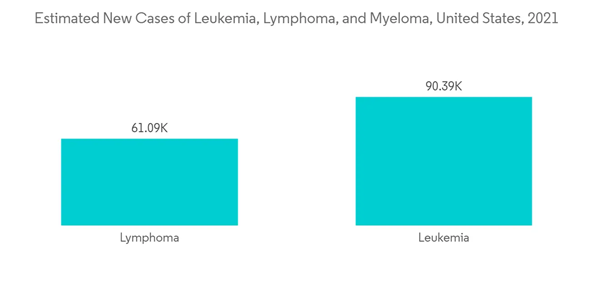The High-content Screening Market is poised to grow at a CAGR of 8.74% over the forecast period.
The outbreak of COVID-19 has turned the spotlight on the high-content screening market as most biopharmaceutical players were engaged in the development of novel therapeutics against the SARS-CoV-2 virus. Due to the increase in drug discovery, there was an increasing demand for drug testing products and services to monitor and evaluate the toxicity of a specific vaccine before commercializing it to the market. Due to this, the pandemic had a positive impact on the high-content screening market. According to the research article published in the American Chemical Society Pharmacology and Translational Science in 2020, new potential therapeutics for COVID-19 identified with a combined virtual and experimental screening strategy and selected from among the already approved drugs were studied to screen for structural similarity against a library of almost 4000 approved drugs with hydroxychloroquine (HCQ) as a reference drug. The study proposed zuclopenthixol, nebivolol, and amodiaquine as potential candidates for clinical trials against the early phase of the SARS-CoV-2 infection and remdesivir and favipiravir therapeutics as potential adjuvants in COVID-19 treatment. With the rising use of high-content screening for such studies, COVID-19 significantly impacted the market studied. The market is expected to show stable growth during the forecast period.
The need for cost containment in pharmaceutical research and development, advancements in informatics solutions and imaging instruments, and government funding and venture capital investments across developed markets are the major factors driving the growth of the High-Content Screening (HCS) Market.
Under the conventional method of toxicity studies, large libraries are screened in search of potential drug candidates. The traditional way is expensive, resource- and time-consuming, and it has a low success rate. Consequent High-Content Screening (HCS) solutions for testing the potential toxicity of chemicals and complex substances are being adopted by pharmaceutical companies to improve in-vitro toxicity testing by reducing the time and cost. The increasing number of contract research organizations providing HCS outsourcing services and the application of HCS in personalized medicine present significant growth opportunities in the market.
The recent technological developments by the market players and researchers, rising investments, and rising research and development activities worldwide are contributing to the growth of the studied market. For instance, in October 2021, BD Life Sciences-Biosciences launched its second Center of Excellence (CoE) in flow cytometry for clinical research in India in collaboration with Christian Medical College, Vellore. The partnership will allow hematologists, doctors, and lab professionals from all over India to deliberate and discuss standardization and best practices in clinical flow cytometry. This CoE will function as the National Reference Center for clinical diagnostics applications and is estimated to propel the High-Content Screening Market during the forecast period.
Furthermore, in April 2022, CytoTronics received USD 9.25 million in initial seed funding led by Anzu Partners, with participation from Milad Alucozai (BoxOne Ventures) and institutional investors. This funding will allow the company to commercialize its research-demonstrated devices for cell-based drug screening in multiple areas, such as oncology, gastroenterology, cardiology, and neurology. It is likely to expand its offerings in terms of bringing technologically advanced products to the market for drug screening purposes which is expected to propel the High-Content Screening Market during the forecast period.
Hence, with the factors such as rising healthcare investment, and increasing strategic initiatives by key players, it is believed that the market studied will witness healthy growth over the forecast period.
Various studies are done that deploy flow cytometry in research laboratories which are expected to propel the segment utility. Its primary users are immunologists and hematologists, who use it for cell sorting and analysis. For instance, a research study published in Frontiers in Immunology in February 2022, was conducted to develop a flow cytometric procedure for quantitative expression profiling of surface antigens on blood leukocyte subsets that are standardized across multiple research laboratories. The process, bioinformatics pipeline, and improved flow panels enable benchmarking new antibody clones to established CD markers and mapping the expression patterns of mAb clones with HLDA approval to CD markers. The advantages of flow cytometry, like the high-speed quantitative and high-content analysis of cells, make the technique an attractive technology for drug discovery applications. The rising research studies using flow cytometry and its advantages are expected to propel the segment growth during the analysis period.
Various market players are engaged in strategic initiatives such as product launches, partnerships, and innovation in technology to develop an advanced solution in the flow cytometry segment. For instance, in March 2022, Beckman Coulter Life Sciences launched the CellMek SPS solution to manual sample preparation and data management bottlenecks in clinical flow cytometry. The fully automated sample preparation system (SPS) offers on-demand processing to assist laboratories in enhancing their capabilities. Such launches are expected to propel the segment growth during the forecast period.
Therefore, flow cytometry is becoming an ideal tool, particularly in an environment where primary cell-based assays are increasingly being deployed to monitor drug responses. The increasing use of phenotypic cell-based assays in drug discovery and the need to monitor drug responses are major factors for the growth of flow cytometry. Therefore, the usage is expected to increase in the coming years. Additionally, the utilization of flow cytometry in the detection of cancer is further augmenting the growth of the segment.
According to the American Cancer Society’s 2022 report, about 1,918,030 new cancer cases were estimated in 2022 in the United States. Also, according to the Canadian Cancer Statistics November 2021 report, an estimated 2 in 5 Canadians were likely to be diagnosed with cancer in their lifetime. It stated that an estimated 229,200 Canadians was predicted to be diagnosed with cancer in 2021. Thus, the high prevalence of cancer is expected to boost the high-content screening market in the region for providing the best safety and efficacy to the manufactured drugs.
The increasing number of cardiovascular diseases among patients further increases the need and demand for effective therapeutics for various inherited cardiovascular conditions. For instance, the prevalence rate of heart failure in the United States is 6 million, which is 1.8% of its total population, as reported by the American Heart Association in 2021. This is expected to be one of the major driving factors for the growth of the market studied in the United States.
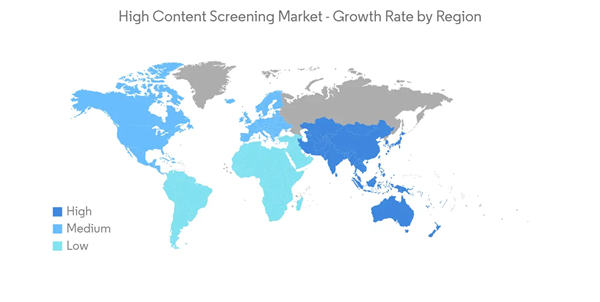
Therefore, owing to the factors mentioned above, such as the high prevalence of cancer and chronic diseases boosting the demand for high-content screening for boosting drug efficacy, the high-content screening market is expected to witness substantial growth in North America during the forecast period.
This product will be delivered within 2 business days.
The outbreak of COVID-19 has turned the spotlight on the high-content screening market as most biopharmaceutical players were engaged in the development of novel therapeutics against the SARS-CoV-2 virus. Due to the increase in drug discovery, there was an increasing demand for drug testing products and services to monitor and evaluate the toxicity of a specific vaccine before commercializing it to the market. Due to this, the pandemic had a positive impact on the high-content screening market. According to the research article published in the American Chemical Society Pharmacology and Translational Science in 2020, new potential therapeutics for COVID-19 identified with a combined virtual and experimental screening strategy and selected from among the already approved drugs were studied to screen for structural similarity against a library of almost 4000 approved drugs with hydroxychloroquine (HCQ) as a reference drug. The study proposed zuclopenthixol, nebivolol, and amodiaquine as potential candidates for clinical trials against the early phase of the SARS-CoV-2 infection and remdesivir and favipiravir therapeutics as potential adjuvants in COVID-19 treatment. With the rising use of high-content screening for such studies, COVID-19 significantly impacted the market studied. The market is expected to show stable growth during the forecast period.
The need for cost containment in pharmaceutical research and development, advancements in informatics solutions and imaging instruments, and government funding and venture capital investments across developed markets are the major factors driving the growth of the High-Content Screening (HCS) Market.
Under the conventional method of toxicity studies, large libraries are screened in search of potential drug candidates. The traditional way is expensive, resource- and time-consuming, and it has a low success rate. Consequent High-Content Screening (HCS) solutions for testing the potential toxicity of chemicals and complex substances are being adopted by pharmaceutical companies to improve in-vitro toxicity testing by reducing the time and cost. The increasing number of contract research organizations providing HCS outsourcing services and the application of HCS in personalized medicine present significant growth opportunities in the market.
The recent technological developments by the market players and researchers, rising investments, and rising research and development activities worldwide are contributing to the growth of the studied market. For instance, in October 2021, BD Life Sciences-Biosciences launched its second Center of Excellence (CoE) in flow cytometry for clinical research in India in collaboration with Christian Medical College, Vellore. The partnership will allow hematologists, doctors, and lab professionals from all over India to deliberate and discuss standardization and best practices in clinical flow cytometry. This CoE will function as the National Reference Center for clinical diagnostics applications and is estimated to propel the High-Content Screening Market during the forecast period.
Furthermore, in April 2022, CytoTronics received USD 9.25 million in initial seed funding led by Anzu Partners, with participation from Milad Alucozai (BoxOne Ventures) and institutional investors. This funding will allow the company to commercialize its research-demonstrated devices for cell-based drug screening in multiple areas, such as oncology, gastroenterology, cardiology, and neurology. It is likely to expand its offerings in terms of bringing technologically advanced products to the market for drug screening purposes which is expected to propel the High-Content Screening Market during the forecast period.
Hence, with the factors such as rising healthcare investment, and increasing strategic initiatives by key players, it is believed that the market studied will witness healthy growth over the forecast period.
High Content Screening Market Trends
Flow Cytometry is Expected to Hold a Significant Market Share Over The Forecast Period
Flow cytometry is used in many research and clinical laboratories. Previously, flow cytometry was significantly restricted because of its limitations in handling scores of samples. However, with unparalleled advances in cell-based analysis and screening in recent years, this technique has been utilized as a powerful screening tool. Various studies are done that deploy flow cytometry in research laboratories which are expected to propel the segment utility. Its primary users are immunologists and hematologists, who use it for cell sorting and analysis. For instance, a research study published in Frontiers in Immunology in February 2022, was conducted to develop a flow cytometric procedure for quantitative expression profiling of surface antigens on blood leukocyte subsets that are standardized across multiple research laboratories. The process, bioinformatics pipeline, and improved flow panels enable benchmarking new antibody clones to established CD markers and mapping the expression patterns of mAb clones with HLDA approval to CD markers. The advantages of flow cytometry, like the high-speed quantitative and high-content analysis of cells, make the technique an attractive technology for drug discovery applications. The rising research studies using flow cytometry and its advantages are expected to propel the segment growth during the analysis period.
Various market players are engaged in strategic initiatives such as product launches, partnerships, and innovation in technology to develop an advanced solution in the flow cytometry segment. For instance, in March 2022, Beckman Coulter Life Sciences launched the CellMek SPS solution to manual sample preparation and data management bottlenecks in clinical flow cytometry. The fully automated sample preparation system (SPS) offers on-demand processing to assist laboratories in enhancing their capabilities. Such launches are expected to propel the segment growth during the forecast period.
Therefore, flow cytometry is becoming an ideal tool, particularly in an environment where primary cell-based assays are increasingly being deployed to monitor drug responses. The increasing use of phenotypic cell-based assays in drug discovery and the need to monitor drug responses are major factors for the growth of flow cytometry. Therefore, the usage is expected to increase in the coming years. Additionally, the utilization of flow cytometry in the detection of cancer is further augmenting the growth of the segment.
North America is Expected to North America is Expected to Hold a Significant Market Share Over The Forecast Period
North America is expected to have a significant market share over the forecast period in the high-content screening market due to the growing research and development activities by pharmaceutical companies, the growing prevalence of chronic diseases in the country, and increasing technological advancements in high-content screening methods.According to the American Cancer Society’s 2022 report, about 1,918,030 new cancer cases were estimated in 2022 in the United States. Also, according to the Canadian Cancer Statistics November 2021 report, an estimated 2 in 5 Canadians were likely to be diagnosed with cancer in their lifetime. It stated that an estimated 229,200 Canadians was predicted to be diagnosed with cancer in 2021. Thus, the high prevalence of cancer is expected to boost the high-content screening market in the region for providing the best safety and efficacy to the manufactured drugs.
The increasing number of cardiovascular diseases among patients further increases the need and demand for effective therapeutics for various inherited cardiovascular conditions. For instance, the prevalence rate of heart failure in the United States is 6 million, which is 1.8% of its total population, as reported by the American Heart Association in 2021. This is expected to be one of the major driving factors for the growth of the market studied in the United States.
Therefore, owing to the factors mentioned above, such as the high prevalence of cancer and chronic diseases boosting the demand for high-content screening for boosting drug efficacy, the high-content screening market is expected to witness substantial growth in North America during the forecast period.
High Content Screening Market Competitor Analysis
The Market for High Content Screening (HCS) is moderately competitive. For compound profiling applications, many pharmaceutical companies are partnering with contract research organizations to identify potential candidates for drug discovery. The HCS market is highly competitive in nature. Some of the key companies in the market are Agilent Technologies, Cytiva, BD (Becton, Dickinson and Company), Bio-rad Laboratories, and Thermofisher Scientific, among others.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
1 INTRODUCTION
4 MARKET DYNAMICS
5 MARKET SEGMENTATION (Market Size by Value - USD million)
6 COMPETITIVE LANDSCAPE
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Danaher Corporation
- Perkinelmer Inc.
- Thermo Fisher Scientific Inc.
- BD (Becton, Dickinson and Company)
- Agilent Technologies
- Bio-Rad Laboratories Inc.
- Yokogawa Electric Corporation
- Merck KGaA
Methodology

LOADING...