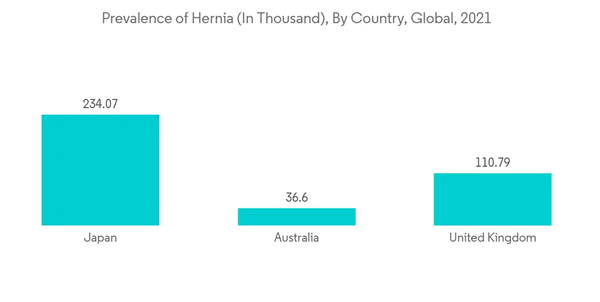
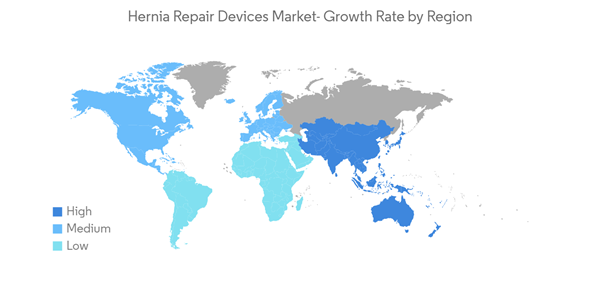
The early phase of the COVID-19 pandemic had an adverse impact on the growth of the market due to the sudden imposition of strict lockdowns across the globe during the pandemic. These circumstances reduced many patient visits and canceled hernia surgery which resulted in complications in the operated patients during the COVID-19 elective surgery cancellation. As per the article published by Surgery Journal in May 2022, 43% of patients underwent inguinal hernia repair during the COVID-19 elective surgery cancellation, 6.6% experienced incarceration and 15.2% had an emergency department encounter. Thus, the COVID-19 outbreak had an adverse impact due to the cancellations of hernia surgeries. However, it is anticipated that the easing of limitations and resumed surgical treatments in the post-pandemic period will accelerate the market's growth during the next few years.
The growth of the global hernia repair devices market is majorly driven by an increase in the prevalence of hernia cases, especially inguinal hernia. Other factors driving the market's expansion include a rise in the use of tension-free repair techniques, technological advancements made by major companies in mesh materials like biological mesh, and an increase in demand for robotic surgery. For instance, as per the article published by Wolters Kluwers in April 2022, the most frequent surgical ailment seen by primary care physicians is groin hernias, which account for 500,000 surgical procedures and 1.6 million annual diagnoses in the United States. Hence, a high burden of hernia cases increases the demand for hernia repair devices and boosts market to grow.
Furthermore, the establishment of a new hernia center is likely to expand the hernia repair services using the devices. For instance, in December 2022, Orlando health launched a complex hernia center to connect patients with surgeons who can provide a comprehensive, personalized treatment plan for hernia repair using devices and is expected to drive the market over the forecast period.
Thus, the increasing number of hernia cases and surgeries along with new hernia care centers expand hernia care which increases the demand for hernia repair devices and drives the market over the forecast period. However, factors such as the high cost of hernia repair surgeries and inconsistent reimbursement policies, and lack of skilled professionals for operating hernia repair devices hamper the market growth.
Hernia Repair Devices & Procedures Market Trends
Polymeric and Prosthetic Mesh are Expected to Have a Large Share in Market Over the Forecast Period
The prosthetics used for hernia repairs can be non-absorbable, composite, or with an absorbable or non-absorbable barrier. Propylene mesh is the most common type of synthetic hernia mesh which may reduce the chances of a hernia returning. The increasing demand for hernia repair procedures is expected to drive the growth of the studied segment during the forecast period.According to the article published by the Journal of Clinical Medicine in February 2022 polypropylene meshes are considered the gold standard of prosthetic materials used in hernia surgery with the advantage of parietal host tissue in-growth. There is a vast array of prosthetics available for hernia repair.
With new product approvals and launches, the use of hernia mesh solutions for surgically correcting or reconstructing anatomical abnormalities has become widespread. For instance, in April 2022 Ariste Medical received 510(k) clearance from the FDA to market its drug-embedded, synthetic hernia mesh in the United States. Hence, with new product approvals or launches products, there are increases spread of devices throughout the regions which are expected to drive the market over the forecast period through this segment.
Furthermore, in April 2021, Researchers from ICMAB-CSIC and B. Braun Surgical collaborated to develop a bio-based surgical mesh with bio-nanocellulose material and established the results which improve hernia repair surgery.
Thus various advantages, approvals, or launches along with new studies expand the usage of the devices and boost the growth of the market through this segment.
North America is Expected to Dominate the Market Over the Forecast Period
North America has an improving number of healthcare facilities and increasing incidence rates of hernia disorder cases that are expected to drive the overall market. According to the article Inguinal hernia published in March 2021, inguinal hernia repair is a common surgery in the United States. It is estimated that about 800,000 inguinal hernias were performed annually in this region. Similarly, as per the study report published by the Canadian Journal of Surgery in April 2022, 85.6% of inguinal hernia patients were men. Thus with a high burden in the procedures every year, the demand for hernia repair devices increases and is expected to drive the market to grow.Furthermore, the new product developments in North America help to expand the indications in the hernia repair process. For instance, in September 2022, Surgical Technology International released a clinical trial publication stating that non-woven SURGIMESH had significantly reduced post-operative chronic pain and recurrence of the hernia. SURGIMESH is the only non-woven polypropylene matrix mesh that promotes rapid and completes vascularized incorporation in just 12 days. Hence, new product developments in the segment help to expand the indications which are expected to drive the market over the study period.
Thus, all the abovementioned factors like rising hernia repair procedures and new product approvals and launches in the region likely increase the demand for hernia repair devices and are expected to drive the market in the region.
Hernia Repair Devices & Procedures Market Competitor Analysis
The hernia repair market is moderately competitive with several market players. Majority of the hernia repair devices are manufactured by global key players. Market leaders with more funds for research and better distribution system have established their position in the market. Some of the companies which are currently dominating the market are B. Braun SE, Melsungen AG, BD (Becton, Dickinson and Company), Cook Medicals, Coopersurgical, Johnson & Johnson, Insight Medical, Inc, Medtronic, Olympus Corporation, WL Gore & Associates, Abbvie Inc (Allergan Plc).Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- B. Braun SE
- BD (Becton, Dickinson and Company)
- Cook Medicals
- CooperSurgical Inc.
- Johnson & Johnson
- Insightra Medical, Inc
- Medtronic
- Olympus Corporation
- W L Gore & Associates
- Abbvie Inc (Allergan Plc)