COVID-19 is expected to have a significant impact on the growth of the market. The patient registry during COVID-19 was used to register the symptoms of COVID-19 in the patients, the impact of vaccination trials, post-marketing authorization of vaccines, and further research and development activities in innovating vaccines. Furthermore, in August 2020, IQVIA launched its COVID Active Research Experience (CARE) Project at helpstopcovid19.com. The IQVIA CARE Project is an opt-in registry available to anyone to advance understanding of COVID-19 through shared information about disease prevalence, symptom progression, and treatment outcomes. Data science innovations supported by patient registries can provide critical missing pieces to support COVID-19 efforts. Thus, COVID-19 is expected to impact the growth of the market during the forecast period.
Certain factors that are driving the market growth include the implementation of government initiatives to build patient registries, the rising adoption of electronic health records (EHR), and the increasing use of patient registry data for post-marketing surveillance.
Owing to the improved outcomes and quality of care in the 21st century, market players around the world are looking for innovative and cost-effective ways to deliver technology-enabled, patient-centered healthcare solutions, both inside and outside hospital walls. Due to widespread applications, including clinical and non-clinical, electronic health records have been able to attract attention from government sources. Many federal policies, actions, and programs are being initiated by the government of developed and developing countries across the world to experience progress in healthcare services.
The launch of new patient registries across the world and the adoption of key strategies, like mergers and acquisitions, are expected to drive market growth during the study period. For instance, in April 2022, Cerner launched a registry that will collect data from patients diagnosed with Type 2 and Type 3 Gaucher disease, an autosomal recessive disease. It will collect retrospective and prospective clinical and health outcome data for patients diagnosed with Gaucher disease. It is focused on accelerating the discovery, development, and delivery of insights and therapies to improve health.
In February 2022, Canadian Neuromuscular Disease Registry launched a new registry for patients with congenital myasthenic syndrome (CMS). This registry aims to improve access to diagnosis and treatment and develop a Canadian CMS cohort for future research. Thus, such government initiatives boosting the adoption of patient registries will boost market growth.
Thus, due to the above-mentioned factors, the studied market is expected to augment during the study period. However, concerns relating to privacy and data security are inhibiting the growth of the global patient registry software market. Moreover, the lack of trained and skilled professionals is limiting the market.
Patient Registry Software Market Trends
Integrated Software Segment is is Expected to Hold a Major Market Share in the Patient Registry Software Market
Integrated software provides multiple modules offering a comprehensive range of features normally available in stand-alone software. A business software productivity program that incorporates several applications (typically word processing, database management, spreadsheet, graphics, and communications) into one product, allowing data sharing between all or most modules. Integrated software is being increasingly used in hospital settings, where multiple registries need to be recorded in different departments, and cross-sharing of this data across departments is crucial.The article "What Are the Benefits of Clinical Documentation Improvement (CDI)?" published in February 2022, stated the result of a survey stated that 36% of clinicians spend more than half their day on administrative tasks in Electronic Health Records. 72% of clinicians expect the time they spend on administrative tasks to increase over the next 12 months. Due to the expected increase in the required clinical documentation, there will be an increase in demand for integrated patient registry software, which will boost the market growth.
Additionally, the launch of new products in the segment will further drive market growth. For instance, in April 2022, UC San Francisco (UCSF), in collaboration with the Quantum Leap Healthcare Collaborative (QLHC) and the United States Food and Drug Administration (FDA), developed the OneSource system to seamlessly integrate clinical care and research data. OneSource is an electronic data capture (EDC) system that was developed to radically streamline the collection and distribution of patient health data for clinical trials.
In February 2022, EVERSANA and THREAD launched a combined offering for pharmaceutical companies. This solution combines EVERSANA's integrated commercial services, rich electronic medical record (EMR) data, and real-world data (RWD) driven recruitment capabilities with THREAD's leading decentralized clinical trial platform to aggregate and link patient-generated and secondary regulatory-grade RWD. Thus, the adoption of such partnerships and collaborations by major players will help in expanding their product portfolio and contribute to segment growth.
Thus, owing to the above factors, the segment is expected to show growth over the forecast period.
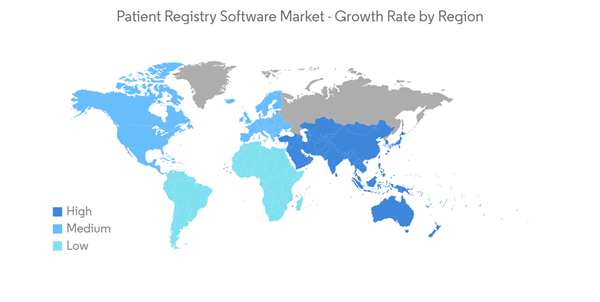
North America is Expected to Hold a Significant Share in the Market and Expected to do Same Over the Forecast Period
North American region is expected to register significant growth during the study period owing to the factors such as the rising adoption of electronic health records, rise in government initiatives for the adoption of patient registry software, and rise in research and development activities by pharmaceutical companies.The United States within the North American region is expected to dominate the market. In the United States, digital infrastructure development for healthcare and electronic health record adoption started earlier. For example, within the country, the federal government has been encouraging the use of its healthcare data through various policies and initiatives.
The article "How A Clinical Data Registry Can Work With Your EHR," published in January 2022, suggests that information in a data registry is used to help clinicians in choosing the most effective course of treatment for patients suffering from the same disease or condition. It also allows clinicians to investigate trends in the patient population in real-time, for instance, seeing if a new therapy is effective or not when compared to existing treatments. Thus, the benefits of a patient registry will increase the adoption of them in the country, thus driving the market.
Additionally, the launch of new products and software in the patient registry market will drive the market. In May 2022, The National Pancreas Foundation ("NPF") launched the pancreatic disease patient registry in partnership with SeqsterOS. The patient-centric registry gives patients and their caregivers the ability to create their longitudinal patient records with real-time data from any EHR, genomic DNA test, and wearable/remote monitoring device.
Moreover, in March 2022, The Rare Diseases Clinical Research Network (RDCRN) launched a contact registry to connect rare disease patients with researchers and advance rare disease research. The registry will collect and maintain the contact information of people who want to receive information about rare disease research. It will also inform participants about opportunities to participate in research. Thus, such launches in the country will help in integrating patient data, which might further help in clinical trials and drive the market.
Thus, due to the above-mentioned factors, the studied market is expected to drive the market in the region during the study period.
Patient Registry Software Industry Overview
The patient registry software market is moderately competitive. Factors such as product launches and the adoption of key strategies by major players will lead the market players to drive the market. Companies, like Dacima Software Inc., FIGmd, Inc., Global Vision Technologies, Inc., Image Trend, Inc., IQVIA, Liasion Technologies, McKesson Corporation, Syneos Health, and Velos Inc., among others, hold a substantial market share in the market studied.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Dacima Software Inc.
- FIGmd Inc.
- Global Vision Technologies Inc.
- Image Trend Inc.
- IQVIA
- Ordinal Data, Inc.
- McKensson Corporation
- Syneos Health
- Velos Inc.
- Cerner Corporation
- IBM
- ArborMetrix




![Patient Registry Software Market by Disease (Diabetes, Cancer, Rare, Asthma, Kidney), Product (Drugs, Device), Use Case (Population Health, Research), End User [(Profit: Pharma, Payer, Hospital), (Non-Profit: Govt)] & Region - Global Forecast to 2030 - Product Image](http://www.researchandmarkets.com/product_images/12796/12796179_60px_jpg/patient_registry_software_market.jpg)



