COVID-19 significantly impacted the zika virus testing market during the forecast period. The spread of infectious diseases increased with the rising prevalence of coronavirus; hence, the demand for testing to diagnose the zika virus also increased during the pandemic. An NCBI study published in October 2021 showed that Zika infection closely resembles COVID-19 and other arboviral infections and stated that there was a considerable risk of Zika Virus Disease spreading across the world, which is especially dangerous in the backdrop of the COVID-19 virus outbreak. Thus, public health measures, vector control, and early diagnosis, especially in the case of pregnant women, are suggested during the pandemic in India to control the spread of the disease. Hence, the similar nature of infection of both the COVID-19 and Zika virus led to an increasing zika virus testing for diagnosis purposes globally, thereby impacting the growth of the market during the pandemic period. Also, the rising focus on the Zika virus diagnosis during the post-pandemic period is expected to contribute to the market's growth over the forecast period.
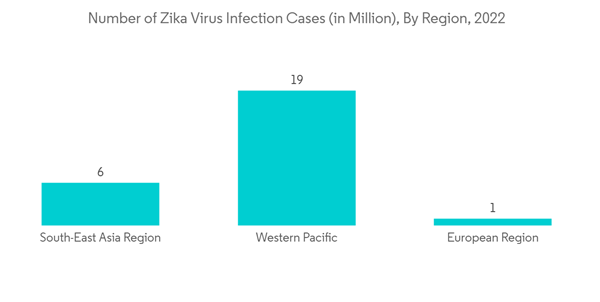
Factors such as the increasing prevalence of zika virus infection and technological advancements in zika virus testing are the major factors driving the growth of the market studied. As per the WHO data in October 2022, Zika virus (ZIKV) infection has been known to be endemic in Africa and Southeast Asia. The consequent viral spread over the Pacific region led to an outbreak in the developed and emerging countries. In addition, there have been major outbreaks of Zika virus infection in Asia-Pacific (particularly India) and South American countries. Also, the WHO data updated in February 2022 stated that Zika transmission persists in several countries but is most common at low levels till 2021. Thus, the rising prevalence of Zika virus infection is one of the primary factors driving the market's growth over the forecast period.
The other prominent drivers of the market studied are increasing research and development by biopharmaceutical companies and technological advancements in diagnostic tests. On the Other hand, governments worldwide are focusing more on initiatives to halt zika virus transmission is driving the studied market growth. For instance, in May 2022, the government of Uttar Pradesh (India) launched the 'Zero Mission' 'Zero to eradicate vector-borne diseases such as Japanese encephalitis, dengue, malaria, typhoid, pneumonia, and Zika virus. Thus, the rising prevalence of Zika virus infection worldwide and government initiatives are among the primary factors driving the market's growth over the forecast period.
However, the excessive cost of testing kits is predicted to hinder market growth in the forecast period.
Zika Virus Testing Market Trends
Molecular Test Segment is Expected to Show Better Growth Over the Forecast Years
Nucleic Acid Amplification Test (NAAT) is a generic term referring to all molecular tests used to detect viral genomic material. The NAAT assays are the preferred method of diagnosis because they can provide confirmed evidence of infection. The increasing demand for tests for zika virus testing has been increasing recently. Hence the molecular test segment is anticipated to witness growth over the forecast period.The increasing research and development in the molecular testing segment are bolstering the growth of the segment. For instance, in March 2022, an international team of researchers headed by experts from the University of Toronto revealed results for one of the first field trials for a synthetic biology-based diagnostic using patient samples. This study was conducted on-site in Latin America and reveals the potential for cell-free synthetic biology tools and companion hardware for providing rapid, decentralized, and low-cost patient testing for infectious diseases like the Zika virus.
The study results published in Nature Biomedical Engineering in March 2022 showed that the novel diagnostic platform has analytical specificity and sensitivity, which is equivalent to the United States Centre for Disease Control PCR test for Zika, and has a diagnostic accuracy of 98.5% with 268 patients samples collected in Recife, Brazil. The platform is also programmable and can be similarly applied to detect any pathogen sequence. Also, the researchers state that on the molecular side, the cell-free tests can be freeze-dried, allowing for distribution without refrigeration. All the molecular components of the test are independent of the PCR-supply chain. Thus, the rising research on molecular testing and its efficacy is bolstering segment growth.
The increasing adoption of molecular Zika virus testing by various government organizations and market players' strategies, such as partnerships and acquisitions, are also expected to contribute to the growth of the studied segment. For instance, in November 2021, the India Molecular Diagnostics and Research Laboratory (MDRL) of the Government Institute of Medical Sciences (GIMS) in Greater Noida, India, started conducting tests for the zika virus. Also, in September 2021, Roche acquired 100% of the shares of TIB Molbiol Group to enhance its molecular diagnostic solutions portfolio.
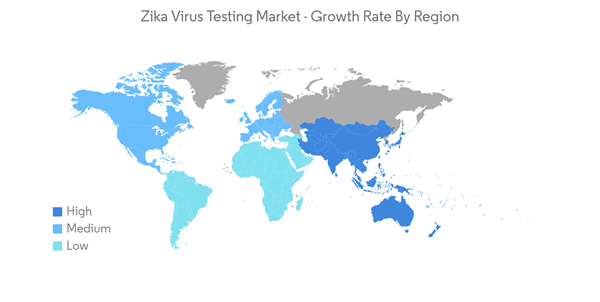
Asia Pacific is Expected to Hold Significant Share in the Market Over the Forecast Period
Asia Pacific is expected to hold a significant share of the market over the forecast period. Factors such as the increasing prevalence of the Zika virus (ZIKV) in countries across Asia-pacific and rising demand for effective testing are expected to contribute to the growth of the market in this region over the forecast period.The prevalence and incidence of the zika virus in India are anticipated to drive the demand for zika virus testing in the country. For instance, the WHO data updated in October 2021 showed that on July 8, 2021, a ZIKV infection was laboratory-confirmed in a resident of Kerala, India. This represented the first Zika virus disease case ever reported from Kerala. Again, on July 31, 2021, Maharashtra state also reported its first Zika laboratory-confirmed case from Belsar village in Pune, India. In addition, Indian health authorities reported a total of 152 Zika virus cases in Uttar Pradesh as of December 5, 2021. The outbreak began in October and represented the first major outbreak identified in the state. Such incidence of zika virus cases in Asia-Pacific countries is expected to contribute to the demand for Zika virus testing, thereby boosting the growth of the market.
Moreover, as per the data provided by the Union Minister of Health and Family Welfare in December 2021, 237 Zika virus cases were reported in December 2021 across India. Hence, the increasing prevalence of the zika virus has created an emergence of zika virus testing in this region, thereby driving the growth of the studied market in this region.
Likewise, serological evidence of widespread Zika Virus has been found in some other countries in Asia-Pacific. An NCBI article published in July 2021 stated that when 997 serum samples were randomly collected from dengue patients and tested for ZIKV, widespread ZIKV exposure was found in the Philippines. The above-mentioned study also suggested that zika virus testing across the country is needed to find the ZIKV infection risk factor. Thus, the spread of the zika virus across countries in Asia-pacific is expected to drive the studied market growth in the region.
Therefore, owing to the factors mentioned above, such as the high prevalence of zika virus infections and the rising demand for effective diagnosis, the zika virus testing market is anticipated to see growth over the forecast period in the Asia-Pacific region.
Zika Virus Testing Industry Overview
The Zika Virus Market is competitive due to the presence of major players in the global market. In the current scenario, the number of product launches, collaborations, and other strategies by the market players is increasing, and it is fueling the Zika Virus Testing Market. Abbott, F. Hoffmann-La Roche Ltd, DiaSorin (Luminex Corporation), Quest Diagnostics, and Siemens Healthcare GmbH are some of the major players, among others.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott
- altona Diagnostics GmbH
- Chembio Diagnostics, Inc.
- ELITechGroup
- F. Hoffmann-La Roche Ltd
- Genekam
- DiaSorin (Luminex Corporation)
- Novacyt Group
- Quest Diagnostics
- Siemens Healthcare GmbH
- LGC Limited (SeraCare Life Sciences)
- Co-Diagnostics, Inc.
- Mediven (Medical Innovation Ventures )