The ongoing Covid-19 pandemic is expected to have a significant impact on the studied market. The market growth was hampered by the decline in sales by major players in the initial phase of the pandemic due to the shutdown and closure of the manufacturing sites. For instance, according to a press release titled "Single-use Lead Times up to 12 Months as COVID Takes Its Toll" by one of the global market players ABEC in May 2021, few manufacturers have waited up to 1 year for single-use equipment, while vendors who are expanding capacities, these projects took months to come online. Thus, the sales of single-use bioreactors were affected by the pandemic. However, single-use bioprocess materials and systems were considered essential resources for the creation and distribution of possible vaccines and treatments for the disease. And, these platforms were used to make innovative vaccines, such as mRNA, DNA, and viral vectors, and were generally constructed using single-use technologies due to the speed and flexibility required. In the article 'COVID-19 Drives Surge in Growth of Single-use Technologies' published in July 2022 it was mentioned that biopharmaceutical companies sought out adaptable technologies that could be swiftly brought online to mass produce vaccines against the SARS-CoV-2 virus. As a result, COVID-19 marked a turning point for single-use systems (SUS), with a sharp rise in demand for reusable and adaptable technology for industrial manufacture. Therefore, such factors mentioned above indicate that the Covid-19 pandemic has had a significant impact on the market studied.
Certain factors that are driving the market's growth include rapid adoption of single-use technologies (SUTs) by the industry, cost-effectiveness, lower downtime, and the launch of new products.
Furthermore, the inclination of the industry toward using singleuse bioreactors (SUBs) is currently being influenced by the criticality of the step, the value of the product, and the time for product development and production. Additionally, as per the article 'Advancing upstream biomanufacturing: continuous perfusion cell culture' published in July 2022 it was mentioned that initial continuous perfusion process development frequently takes more time than batch and fed-batch processes. However, once the procedure is in practice, productivity gains might counterbalance this. These benefits include enabling manufacturers to reach clinical production quantities more readily by allowing for lesser scale-up production operational volume requirements. And these benefits can be achieved using single-use bioreactors. Therefore, such advantages in manufacturing facilities that are gained by using single-use bioreactors are anticipated to contribute to market growth.
In the bioprocessing sector, SUBs has become a standard for both clinical and industrial batches. Thus, significant developments are being made in the single-use bioreactor segment which is also fuelling the market growth. For instance, in March 2021, Thermo Fischer Scientific launched the 3,000 L and 5,000 L HyPerforma DynaDrive single-use bioreactors. The 3,000 L and 5,000 L vessels in this product have the same footprint, with hardware that is simplified and optimized for perfusion cell culture procedures to assist conserve manufacturing suite space. Thus, such developments are anticipated to drive market growth over the forecast period.
Therefore, owing to the above-mentioned factors the market is predicted to witness growth over the forecast period. However, high regular recurring expenses may be involved which is anticipated to hinder the market growth.
Single Use Bioreactor Market Trends
Monoclonal Antibodies Segment is Anticipated to Witness Growth
The main advantage of using monoclonal antibodies (MAbs) in treating several diseases (such as lymphoma and leukemia, as well as autoimmune diseases) is that they are taken from a biological source and can produce antibodies in the long term. While the use of disposables (single-use bioreactors) eliminates cleaning and sterilization steps, as well as cleaning validation, it reduces cost and the time of operation per batch. Thus, with the increasing demand for MAbs manufacturing, the application of single-use bioreactors (SUBs) for the production and manufacturing of MAb is also increasing. Hence, it is expected to drive the growth of the segment.An increase in research and development (R&D) activities, collaborations, and strategic partnerships along with soaring demand for new vaccines and therapeutics and rising chronic and infectious diseases is expected to boost the segment growth. For instance, in January 2022, HaemaLogiX Ltd (HaemaLogiX) and Lonza entered into an agreement to manufacture the next clinical batch (cGMP) of HaemaLogiX's lead multiple myeloma drug candidate, KappaMab, a monoclonal antibody that binds to a cell surface target called kappa myeloma antigen (KMA) that is only found on myeloma cancer cells and not on normal plasma cells. Such strategies for the development of monoclonal antibodies are anticipated to create demand for SUBs used for manufacturing facilities, thereby, expected to drive segment growth.
Moreover, an article published by 53Biologics in July 2022 stated that monoclonal antibody biotherapeutics account for roughly 1/5th of new drug approvals by the Food and Drug Administration (FDA) every year, and bioreactors such as SUBs can improve efficiency and performance in MAb manufacturing. Thus, the improved efficiency and performance of SUBs in manufacturing of MAb are expected to boost the segment growth.
Therefore, owing to the factors mentioned above, the segment is anticipated to witness growth over the forecast period.
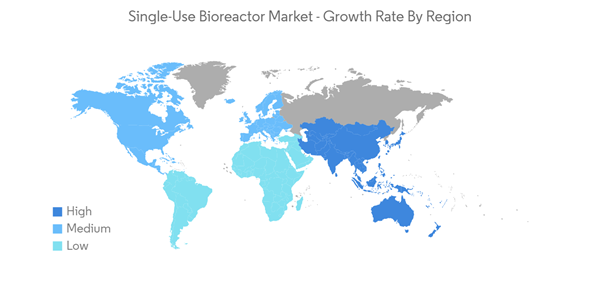
North America Dominates the Market and is Expected to do so during the Forecast Period
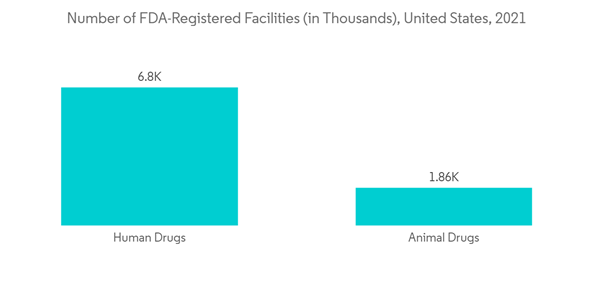
North America is expected to be a dominant region in the single-use bioreactors (SUBs) market due to increasing chronic illnesses coupled with the emerging need for developing new drugs.More than 34.2 million Americans have diabetes, and more than 868,000 people die from heart disease each year, according to data from the Centers for Disease Control and Prevention that was published on January 10th, 2022. In addition to this, the data updated by the Food and Drug Administration (FDA) in 'Fact Sheet: FDA at a Glance' published in November 2021 shows that there are 4,944 FDA-Registered Facilities for the development of biologics and 6,799 for human drugs. Such a high number of manufacturing facilities to produce drugs is expected to create opportunities for the use of SUBs. Thereby, expected to drive market growth in the region.
Furthermore, the pharmaceutical and biopharmaceutical industries' innovative drug development is anticipated to fuel market expansion in this sector. About 382 vaccine candidates were under development as of February 2021, according to the International Federation of Pharmaceutical Manufacturers and Associations' "Facts & Figures Report 2021," of which 24 were in Phase I, 34 were in Phase II, and 23 were in Phase III trials. The biopharmaceutical sector is taking extraordinary, quick steps to get vaccinations to patients. Thus, such increasing demand for the development of biologics in the country is expected to create demand for the use of single-use bioreactors over the forecast period.
Moreover, the developments and product launches are also expected to drive market growth in the country. For instance, in October 2021, the Agilitech bioreactor controller was launched by Agilitech, a United States-based company that provides simultaneous control of up to two single-use bioreactors from 30 L up to 2000 L. The design of the product allows for integration with any brand single-use bioreactor. Such developments in the pharmaceutical and biopharmaceutical businesses are driving market expansion and are anticipated to favorably affect the growth of the studied market in the country during the projected year.
Single Use Bioreactor Industry Overview
The competitive landscape of the single-use bioreactors market covers the business overview, financials, products and strategies, and recent developments by market players regionally and globally. The global single-use bioreactor market is competitive and consists of a number of major players. Companies, like ABEC, Celltainer, Distek Inc., Eppendorf AG, Danaher (Cytiva), Merck KGaA, OmniBRx Biotechnologies, Pall Corporation, Sartorius AG, and Thermo Fisher Scientific, among others, hold substantial shares in the single-use bioreactor market.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- ABEC
- Celltainer
- Distek, Inc.
- Eppendorf SE
- Danaher (Cytiva)
- Merck KGaA
- OmniBRx Biotechnologies
- Pall Corporation
- Sartorius AG
- Thermo Fisher Scientific