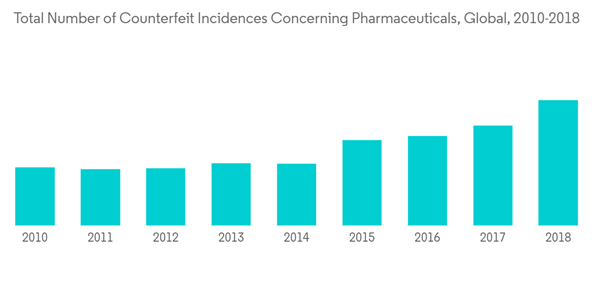
With the development in the COVID-19 vaccine and the various treatment and diagnostic devices and instruments to curb the pandemic, the risk of counterfeit drugs and diagnostic devices increased globally, which impacted the pharmacy automation market. For example, in March and April 2020, Homeland Security in the United States also notified 19,000 suspects of COVID-19-related domain names. As a result, it seized over USD 3.2 million linked to 494 shipments of mislabeled, fraudulent, unauthorized, or prohibited COVID-19 test kits, treatment kits, homeopathic remedies, purported antiviral products, and PPE kits, which further led to 11 arrests. Such instances are expected to drive the adoption of track and trace solutions.
The major factors propelling the growth of the track and trace solutions market include the growth of the medical devices and pharmaceutical industries, an increase in implementation of serialization, a rise in the number of packaging-related product recalls, and an increasing number of counterfeit drugs.
Drug counterfeiting is one of the significant problems in large pharmaceutical and biopharmaceutical companies. As per the article published by the World Health Organization in 2019, about 1 out of 10 medical products that circulate in low- and middle-income countries are found to be substandard or falsified. This factor, consequently, is driving the adoption of track and trace solutions among drug manufacturers and other end users. Furthermore, in December 2020, Janssen Pharmaceutical, a subsidiary of Johnson & Johnson, notified that counterfeit SYMTUZA (darunavir/cobicistat/emtricitabine/tenofovir alafenamide) had been distributed to over three pharmacies in the United States. Janssen is currently working closely with the US Food and Drug Administration (FDA) to prevent further distribution and support the agency's investigation into the reported instances. Hence, these factors are expected to fuel market growth.
However, factors such as high costs associated with serialization and aggregation and lack of common standards for serialization and aggregation may hinder the market growth.
Track and Trace Solutions Market Trends
RFID Technology is Expected to Observe a Good Growth in the Track and Trace Solutions Market
The outbreak of COVID-19 has accelerated digitization, and thereby, the usage of RFID technology in the healthcare sector increased in 2020. These technologies are being used in tracking and authenticating vaccines, diagnostic kits, and other devices. For instance, in June 2020, the United States Department of Health and Human Services, Department of Defense, and ApiJect Systems America signed a partnership for projects, like "Project Jumpstart" and "RAPID USA," for expanding syringe production. Hence, this is expected to support the market growth in this segment due to the COVID-19 situation.The radio-frequency identification (RFID) technology also plays a stellar role in inventory management and supply-chain operations, improving security and product handling. It also reduces the labor and time required for product handling in the supply chain. In addition, it has a huge role in tracking and tracing prescription drugs and reducing billions in costs in the medical and healthcare industry.
As a result, companies are now adopting RFID technology in the production and packaging process. For example, in September 2020, Fresenius Kabi incorporated RFID-tag-equipped labels on medications, such as prefilled syringes and vials, which are commonly used in operating rooms to enhance inventory management workflow. The benefits of using modern and advanced RFID have increased its adoption and acceptance in the healthcare sector, which is critical for boosting the growth of the RFID segment.
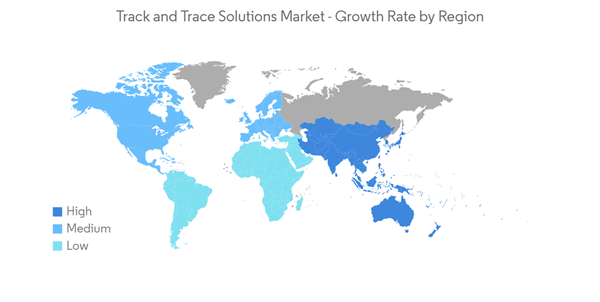
North America is Expected to Dominate the Market Over the Forecast Period
North America currently dominates the market for track and trace solutions and is expected to continue its stronghold over the forecast period. In the North American region, the United States holds the largest market share. However, due to the outbreak of COVID-19, the demand for track and trace solutions have fueled in this region. For instance, in December 2020, Moderna, a clinical-stage biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines to develop a new generation of transformative medicines for patients, notified that Moderna is currently running SAP Digital Supply Chain solutions to help with the serialization and distribution of a potential COVID-19 vaccine. The company presently has mRNA-1273, a vaccine candidate against COVID-19. Therefore, a positive impact is expected on the market due to the COVID-19 pandemic.The primary factor attributing to the market growth is the increasing number of counterfeit drugs. Due to the rising number of counterfeit medicines available in the market, in 2019, the Food and Drug Administration launched a new pilot project to inform the development of a new electronic, interoperable track-and-trace system, as part of the Drug Supply Chain Security Act (DSCSA). This new system will reduce incorrect domestic drug distribution and keep counterfeit drugs from potentially entering the supply chain. The DSCSA pilot program focuses on identifying efficient processes that will comply with drug supply chain security and technology requirements for disseminating information.
Furthermore, according to the United States Food and Drug Administration (FDA), the number of drug recall enforcement issued in the United States was 2,163 in 2019. Also, in July 2019, the US Food and Drug Administration recalled the CapsoCam Plus video capsule system of Capso Vision Inc., due to the mislabeling of the device with incorrect serial number labels, which may have resulted in a patient's misdiagnosis. Such instances have prompted US-based medical devices companies to adopt track and trace solutions.
Moreover, innovative solutions in track and tracing have also boosted this region's market growth. For instance, in June 2020, Zebra Technologies Corp. launched Zebra MotionWorks Proximity, which is featured with proximity sensing at the user level, enabling alerting and contact tracing. This will allow hospitals and other organizations to protect their employees' health while in the work environment. This new product launch by the company will increase its credibility in the market. Therefore, the market studied is expected to propel during the forecast period with all the factors mentioned above.
Track and Trace Solutions Industry Overview
The track and trace solutions market is competitive and consists of several major players. In terms of market share, few major players currently dominate the market. With the rising awareness about track and trace solutions and improving healthcare infrastructure, few other smaller players are expected to enter the market. Some of the major players in the market are Axway, Mettler-Toledo International Inc., Rfxcel Corporation, Sea vision SRL, and TraceLink Inc.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- ACG
- Adents
- ANTARES VISION SpA
- Axway
- Mettler-Toledo International Inc.
- Rfxcel Corporation
- Optel Group
- SEIDENADER MASCHINENBAU GMBH (MEDIPAK SYSTEMS)
- Sea Vision SRL
- TraceLink Inc.
- Syntegon Technology GmbH
- Zetes
- Kezzler AS
- Korber Medipak Systems GmbH