The Growing Adoption of Third-Party Quality Controls is to Drive the Market
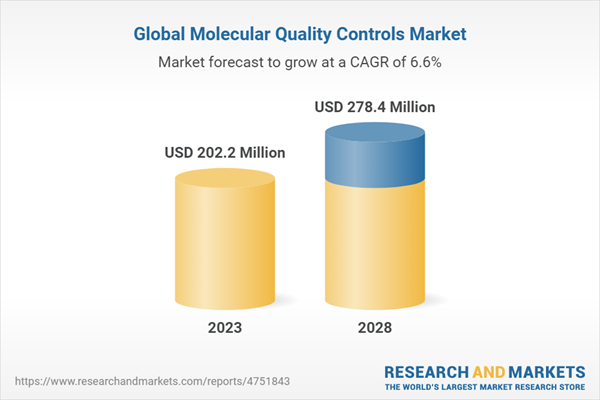
The global molecular quality controls market is projected to reach USD 278.4 million by 2028 from USD 202.2 million, at a CAGR of 6.6% during the forecast period. Growth in this market is primarily driven by the increasing government funding for genomic projects, increasing demand for personalized medicines and declining costs of sequencing procedures, and increasing prevalence of infectious diseases, cancer, and genetic diseases. However, the additional costs involved in the quality control process and budget constraints in hospitals and laboratories, and the unfavorable reimbursement scenario for molecular tests are the major factors that are expected to restrain the growth of this market during the forecast period.
The independent controls segment has accounted for the biggest share of the market during the forecast period.
By product, the molecular quality controls market is segmented into independent controls and instrument-specific controls. The independent controls segment accounted for the biggest share of the global molecular quality controls market in 2022. The increasing use of third-party independent quality controls is responsible for a large share of this segment.
The single-analyte controls have accounted for the biggest share of the market during the forecast period.
By analyte type, the molecular quality controls market is divided into single-analyte controls and multi-analyte controls. Single-analyte controls accounted for the biggest share of the molecular quality controls market in 2022. Factors such as the significant use of singleplex assays in hospitals, and low risk of cross-reactivity are responsible for the large share of this segment.
The infectious disease diagnostic segment accounted for the biggest share of the market in 2022.
By application, the molecular quality controls market is divided into infectious disease diagnostics, genetic testing, oncology testing, and other applications (including neurology disease testing, DNA fingerprinting, tissue typing, microbiology and cardiovascular disease testing). The infectious disease diagnostics segment accounted for the biggest share of the global molecular quality controls market in 2022. The significant increase in the prevalence of infectious diseases is responsible for the large share of this segment.
The diagnostic laboratories segment accounted for the biggest share of the market in 2022.
By end users, the molecular quality controls market is segmented into academic & research institutes, diagnostic laboratories, IVD manufacturers & CROs, hospitals, and other end users (home health agencies, blood banks, nursing homes, and local public health laboratories). The diagnostic laboratories segment accounted for the biggest share of the molecular quality controls market in 2022. The growing number of laboratory tests performed in diagnostic laboratories is responsible for the large share of this segment.
North America accounted for the largest share of the Molecular Quality Controls market in 2022.
Based on region, the molecular quality controls market is segmented into North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa. North America dominated the molecular quality controls market in 2022. The large share of this regional segment is mainly due to the developed healthcare system in the US and Canada, the presence of many leading molecular quality control product manufacturers, and easy accessibility to technologically advanced products in North America.
The break up of the profile of primary participants in the molecular Quality controls market:
- By Company Type: Tier 1- 35%, Tier 2- 45%, and Tier 3-20%
- By Designation: C-level Executives- 35%, Directors- 25, and Others- 40%.
- By Region: North America - 40%, Europe - 30%, APAC -20%, Latin America - 5%,Middle East & Africa-5%
Key players in the molecular quality controls market
The key players operating in the molecular quality controls market include Microbiologics, Inc. (US), Steck, Inc. (US), Bio-Rad Laboratories, Inc. (US), Anchor Molecular (US), Thermo Fisher Scientific, Inc. (US), Randox Laboratories Ltd. (UK), LGC Limited (UK), Abbott Laboratories (US), Fortress Diagnostics (UK), SERO AS (Norway), Anchor Molecular (US), Vircell S.L. (Spain), Ortho Clinical Diagnostics, Inc. (US), F. Hoffmann-La Roche Ltd. (Switzerland), Danaher Corporation (US), Microbix Biosystems Inc. (Canada), Molbio Diagnostics Pvt. Ltd. (India), QuidelOrthoCorporation (US), Sun Diagnostics, LLC (US), Seegene Inc. (South Korea), ZeptoMetrix, LLC (US), Qnostics (UK), Bio-Techne Corporation (US), Helena Laboratories Corporation (US), SpeeDx Pty. Ltd. (Australia), Maine Molecular Quality Controls, Inc. (US), and Grifols, S.A. (Spain).
Research Coverage:
The report analyzes the molecular quality controls market and aims at estimating the market size and future growth potential of this market based on various segments such as product, analyte type, application, and end user. The report also includes a product portfolio matrix of various molecular quality control products available in the market. The report also provides a competitive analysis of the key players in this market, along with their company profiles, service offerings, and key market strategies.
Reasons to Buy the Report
The report will enrich established firms as well as new entrants/smaller firms to gauge the pulse of the market, which in turn would help them, garner a more significant share of the market. Firms purchasing the report could use one or any combination of the below-mentioned strategies to strengthen their position in the market.
This report provides insights into the following pointers:
- Analysis of key drivers (growing adoption of third-party quality controls, increasing investments in genomics, growing preference for personalized medicines, rising demand for external quality assessment support, and increasing prevalence of infectious diseases and cancer), restraints (high costs and budget constraints in clinical laboratories, and unfavorable reimbursement for molecular tests), opportunities (rising demand for multi-analyte controls, and growth opportunities in emerging countries), and challenges (changing regulatory framework)
- Product Development/Innovation: Detailed insights on upcoming trends, research & development activities, and new product launches in the global molecular quality controls market
- Market Development: Comprehensive information on the lucrative emerging markets by product, analyte type, application, and end user.
- Market Diversification: Exhaustive information about new services or service enhancements, growing geographies, recent developments, and investments in the global molecular quality control market.
- Competitive Assessment: In-depth assessment of market shares, growth strategies, product offerings, company evaluation quadrant, and capabilities of leading players in the global molecular quality controls market. This report also helps stakeholders understand the pulse of the molecular quality controls market and provides them with information on key market drivers, restraints, challenges, and opportunities.
Table of Contents
Companies Mentioned
- Abbott Laboratories
- Anchor Molecular
- Bio-Rad Laboratories, Inc.
- Bio-Techne Corporation
- Danaher Corporation
- F. Hoffmann-La Roche Ltd.
- Fortress Diagnostics
- Grifols, S.A.
- Helena Laboratories Corporation
- LGC Limited
- Maine Molecular Quality Controls, Inc.
- Microbiologics, Inc.
- Microbix Biosystems
- Molbio Diagnostics Pvt. Ltd.
- Qnostics
- Quidelortho Corporation
- Randox Laboratories Ltd.
- Seegene Inc.
- Sero AS
- Speedx Pty. Ltd.
- Streck LLC
- Sun Diagnostics, LLC
- Thermo Fisher Scientific, Inc.
- Vircell, S.L.
- Zeptometrix, LLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 268 |
| Published | February 2022 |
| Forecast Period | 2023 - 2028 |
| Estimated Market Value ( USD | $ 202.2 Million |
| Forecasted Market Value ( USD | $ 278.4 Million |
| Compound Annual Growth Rate | 6.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |