Global Duchenne Muscular Dystrophy Drugs Market - Key Trends & Drivers Summarized
What Is Duchenne Muscular Dystrophy and Its Impact?
Duchenne Muscular Dystrophy (DMD) is a severe genetic disorder characterized by progressive muscle degeneration and weakness. It primarily affects boys, with symptoms typically appearing between the ages of two and five. The disease is caused by mutations in the dystrophin gene, which is essential for muscle function. Without dystrophin, muscle fibers are damaged and eventually replaced by fat and connective tissue. The progression of DMD leads to loss of ambulation, respiratory and cardiac complications, and significantly reduced life expectancy. The impact on patients and their families is profound, necessitating ongoing medical care and support.How Are Treatment Approaches Evolving?
Treatment approaches for Duchenne Muscular Dystrophy are evolving rapidly, with advancements in genetic therapies, molecular drugs, and symptomatic treatments. Historically, corticosteroids have been the mainstay of DMD treatment, helping to slow muscle degeneration. However, recent years have seen significant progress in gene therapy and exon-skipping drugs, which aim to address the underlying genetic causes of DMD. These innovative therapies are designed to restore or replace the defective dystrophin gene, offering the potential for more effective and long-lasting treatment outcomes. Additionally, supportive therapies such as physical therapy, respiratory care, and cardiac management remain crucial for improving quality of life and managing disease complications.What Role Do Clinical Trials and Regulatory Approvals Play?
Clinical trials and regulatory approvals are pivotal in the development and availability of new treatments for Duchenne Muscular Dystrophy. Rigorous clinical trials are essential for demonstrating the safety and efficacy of new therapies. These trials often involve multiple phases and require collaboration between researchers, healthcare providers, and patients. Regulatory bodies, such as the FDA and EMA, play a critical role in evaluating and approving new drugs, ensuring that they meet stringent safety and effectiveness standards. Accelerated approval pathways and orphan drug designations are often utilized to expedite the development of treatments for rare diseases like DMD, providing patients with earlier access to promising therapies.What Factors Are Driving Market Growth?
The growth in the Duchenne Muscular Dystrophy drugs market is driven by several factors, including advancements in genetic research, increased funding and investment, and the rising prevalence of the disease. Technological innovations in gene editing and molecular biology are paving the way for more targeted and effective treatments. Increased funding from governments, non-profit organizations, and pharmaceutical companies is accelerating research and development efforts. The rising awareness and diagnosis of DMD are also contributing to market growth, as more patients seek treatment. Additionally, collaborations between biotech firms and academic institutions are fostering the development of innovative therapies. The regulatory environment, with initiatives to fast-track the approval of orphan drugs, is further supporting market expansion, providing hope for improved outcomes for patients with DMD.Report Scope
The report analyzes the Duchenne Muscular Dystrophy Drugs market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below.- Segments: Segment (Exon Skipping, Steroid Therapy, Mutation Suppression, Other Segments).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Hospital Pharmacies segment, which is expected to reach US$16.5 Billion by 2030 with a CAGR of 35.9%. The Drug Store & Retail Pharmacies segment is also set to grow at 40.5% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.8 Billion in 2024, and China, forecasted to grow at an impressive 43.6% CAGR to reach $2.4 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Duchenne Muscular Dystrophy Drugs Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Duchenne Muscular Dystrophy Drugs Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Duchenne Muscular Dystrophy Drugs Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as FibroGen, Inc., ITALFARMACO S.p.A., NS Pharma Inc., PTC Therapeutics, Inc., Sarepta Therapeutics, Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 38 companies featured in this Duchenne Muscular Dystrophy Drugs market report include:
- FibroGen, Inc.
- ITALFARMACO S.p.A.
- NS Pharma Inc.
- PTC Therapeutics, Inc.
- Sarepta Therapeutics, Inc.
- Satellos Bioscience, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- FibroGen, Inc.
- ITALFARMACO S.p.A.
- NS Pharma Inc.
- PTC Therapeutics, Inc.
- Sarepta Therapeutics, Inc.
- Satellos Bioscience, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 220 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
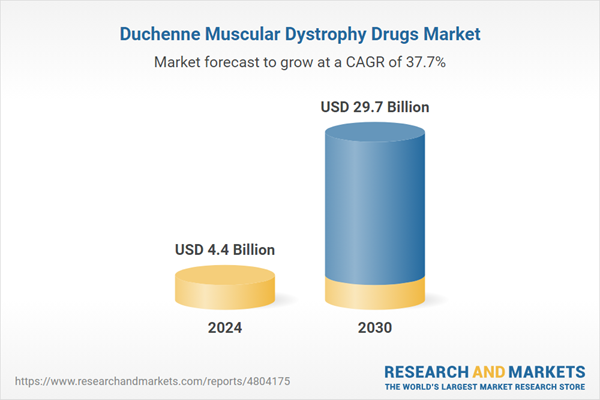
| Estimated Market Value ( USD | $ 4.4 Billion |
| Forecasted Market Value ( USD | $ 29.7 Billion |
| Compound Annual Growth Rate | 37.7% |
| Regions Covered | Global |